ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Single-prolonged stress induces increased caspase-3 and 9 in mPFC in a rat model of post-traumatic stress disorder
1Department of Histology and Embryology, Basic Medical Sciences College, China Medical University, 92 North 2nd Road, Shenyang 110001, PR China
2Institute of Pathology and Pathophysiology, China Medical University, 92 North2nd Road, Shenyang 110001, PR China
3Department of Neurology, the North Hospital, Shenyang, Liaoning 110016, PR China
- *Corresponding Author:
- Yuxiu Shi
Department of Histology and Embryology, Basic Sciences College
China Medical University, PR China
Accepted date: July 29, 2016
The aim of this study was to provide a novel insight into the mechanism of how medial prefrontal cortex (mPFC) participates in Post-Traumatic Stress Disorder (PTSD) by investigating the level of caspase- 3 and 9 in mPFC of Single Prolonged Stress (SPS) rats. A total of 100 male Wistar rats were randomly divided into a normal control group and SPS groups with 1, 4, 7 and 14 days of treatment. The PTSD model was created by SPS. The expressions of caspase-3 in SPS groups were significantly increased, compared with normal control group (P<0.05), and peaked at 7 days after exposure to SPS. In normal control group, the fluorescent intensity of caspase-9 positive cells was poor, and that of SPS groups was significantly stronger than normal control group (P<0.01), and peaked at 4 day after exposure to SPS. After SPS stimulation, caspase-3 and caspase-9 mRNA levels gradually increased, compared with control group, and peaked with 7 and 4 days of treatment, respectively (P<0.01). These results reveal that caspase-9 and caspase-3 may play an important role in the pathology of PTSD, which provides experimental evidence for the pathogenesis of PTSD.
Keywords
Caspase-3, Caspase-9, Mmedial prefrontal cortex, Single-prolonged stress, Posttraumatic stress disorder.
Introduction
Post-Traumatic Stress Disorder (PTSD), an anxiety disorder in which an individual’s ability is impaired by emotional response to memories of a traumatic event, develops after exposure to a life-threatening traumatic experience [1,2]. It is characterized by symptoms that often endure for years including continuous re-experience of the traumatic event, avoidance of stimuli associated with the trauma, numbing of general responsiveness, and increased arousal [3-6]. The pathophysiology of PTSD has been widely studied in neuroscience [7]. However, the mechanism of PTSD is not fully understood until now.
Many lines of evidence from researches for both human and animal now exist to suggest that amygdala; hippocampus and medial prefrontal cortex (medial prefrontal cortex, mPFC) play an important role in PTSD related symptoms [8]. Amygdala has been documented to play an important role in fear, rage, and emotional memory [9,10]. The mPFC has a direct synaptic connection to amygdala and could inhibit amygdala activity [11]. The current study shows that the function of mPFC in patients with PTSD degenerate [12]. In this study, we aim to explore the change of caspase 3 and caspase 9 in the mPFC in order to provide the experimental evidence for degenerate of mPFC whether caused by the change of caspase 3 and caspase 9. Single-prolonged stress (SPS) is a good animal model of PTSD based on the timedependent dysregulation of the HPA axis, and has been developed and employed for PTSD research [13-15]. Many lines of evidence have implicated that medial prefrontal cortex (mPFC) plays an important role in modulating HPA responses to emotional stress [16,17]. Thus, the mPFC have been paid a lot of attention to reveal the mechanism of PTSD.
Very little is currently known regarding caspases in brain, particular in mPFC, we examined the change of caspase 3 and caspase 9 in the mPFC of SPS rat in this study in order to provide experimental evidence for further study related to the pathogenesis of PTSD.
Materials and Methods
Animals
One hundred healthy male Wistar rats aged 7-8 weeks at the start of the study, weighing approximately 180 g, supplied by the Animal Experimental Center, China Medical University, were used for all experiments. All rats were reared in the experimental animal facility for a week to acclimate to new environment (22 ± 1°C and 55 ± 5% humidity) on a 12 h light/ dark schedule with free access to food and water. Experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. All efforts were made to reduce the number of animals used and to minimize animal suffering during the experiment.
Animal model and experimental groups
The SPS model was created, which was described in “Advances in Basic and Clinical Research” international conference launched by Japanese Ministry of Education in 2005. Briefly, rats were restrained for 2 h in an animal holder, and were followed immediately by 20 min of forced swimming (temperature: 25°C, depth: 40 cm). The rats were allowed to recuperate for 15 min. They were then exposed to ether vapor until loss of consciousness. The rats were put back into their home cage and left undisturbed until they were killed for experiments. The rats were randomly assigned to one of five groups of twenty: the SPS treatment groups (1 d, 4 d, 7 d and 14 d) and the normal control group. The normal control rats lived in their individual home cages with no handling, and were killed after acclimating to their new environment for a week.
Brain Tissue Preparation and Immunohistochemical Analysis of caspase 3
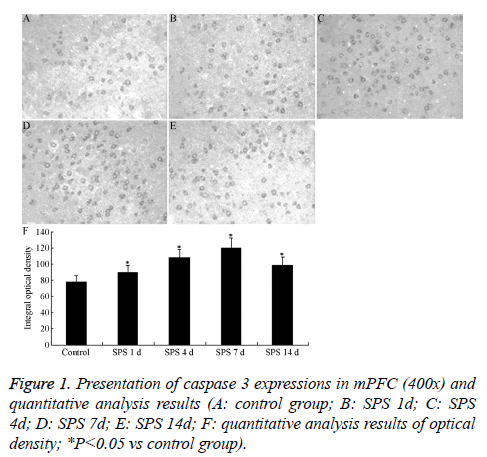
Rats of each group were transcardially infused with 200-300 mL of pre-cold saline through the ascending aorta, followed by 300 mL of 4% pre-cold paraformaldehyde. The whole brains were rapidly removed and dissected on ice, followed by 6-10 h of post-fixation in 4% paraformaldehyde at 4°C. The brain tissue was cut into slices with 15 μm in thickness. After treated with 1% hydrogen peroxide/methanol, the sections were incubated with 10% normal goat serum for 30 min at 37°C, the I antibody (rabbit polyclonal anti-caspase 3 antibody, Cell Signaling, Beverly, MA, USA, 1:300 dilution) at 4°C overnight, and the II antibody (goat polyclonal anti-rabbit IgG, Boster Biological Technology Ltd, China, 1:200 dilution) for 1 h at 37°C. Then, the sections were incubated with avidin-biotin peroxidase coplex (Boster Biological Technology Ltd., Wuhan, China, 1:200 dilution) for 1 h at 37°C and immunocomplexes were finally visualized with 0.05% DAB. An independent investigator obtains images from five slices for each rat and results were analysized by an image analyzer. The average number of positive cells from the amygdala in each rat, located according to the atlas of Paxinos and Watson [18], was a calculated average of three sequential brain slices throughout the area of interest and measured both in the left and right hemisphere. The counting of positive cells was performed by an individual blind to the treatment conditions, using the same magnification and identical color scale setting as a correction for background staining.
Immunofluorescence analysis of caspase-9
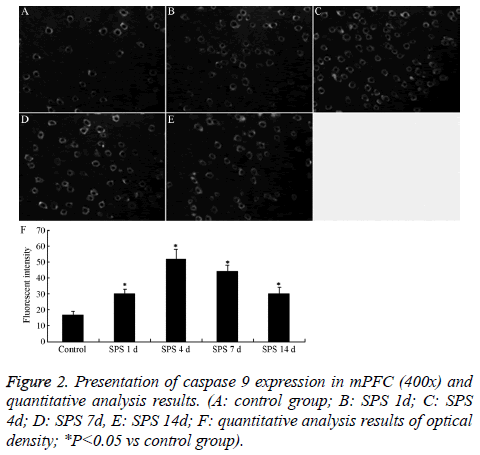
The sections were treated with 5% bovine serum albumin (BSA), 0.3% Triton X-100 in PBS for 30 min to block nonspecific staining at room temperature (RT). Endogenous peroxidase was inactivated with 3% H2O2 in ddH2O for 5 min at room temperature. peronopathol 2004; 99(3-4): 203-13.tissues., conventional pigs at different ages detected by TUNEL and cleaved caspa The sections were then incubated with mouse anti-caspase 9 monoclonal antibody (Santa Cruz, USA; 1:300) in 2% BSA-PBS overnight at 4°C. After being washed with PBS for three times, the sections were incubated with FITC goat anti-mouse IgG (Boster, China; 1:50) for 2 h at room temperature. To assess nonspecific staining, a few sections in every experiment were incubated in buffer without primary antibody. Slices were then mounted with glycerin and observated by fluorescence microscope. Fifteen slides were randomly selected from each group. Each slide was randomly selected five visual fields in mPFC (400x). We recorded the optical density (OD) of caspase 9-immunopositive cells in each field to evaluate the average of OD, and the OD was further analyzed using the MetaMorph/DPIO/BX41 morphology image analysis system.
Reverse transcription-polymerase chain reaction (RTPCR) to detect caspase-3 and caspase-9
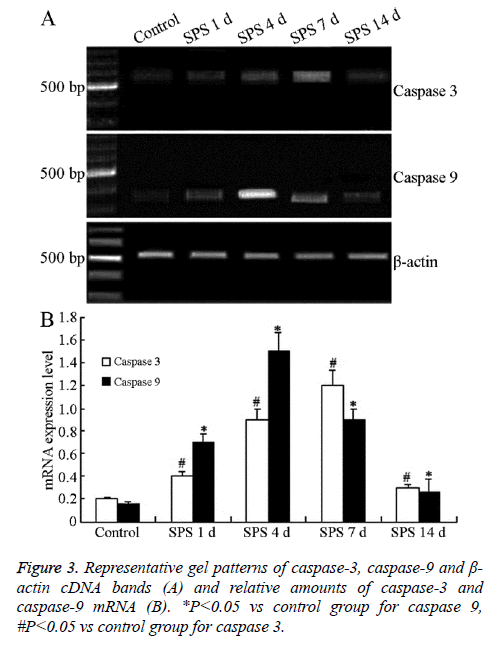
Tissues from the rats of each group were obtained as above and were quickly frozen in liquid nitrogen and stored -80°C. Total mRNA of each group was extracted from the basolateral amygdala according to the instructions of Trizol kit (Invitrogen, USA) and 1 μg of total RNA was reverse transcribed into cDNA. cDNA was amplified using a RNA PCR kit (AM Ver. 3.0, Takara Bio, Otsu, Japan). The primers were designed and synthetized by Shenggong Biotech Company (Shanghai, China) according to the serial number from Genbank were shown in Table 1. The reaction was started at 94°C for 2 min and amplifiied of 40 cycles of 30 s at 94°C, 60 s at 52°C (for caspase-3) or 45 s at 67°C (for caspase-9), 45 s at 72°C and ended with 7 min extension at 72°C. β-actin mRNA used as an internal control was co-amplified with caspase 3- or caspase 9- mRNA. The products were observed after electrophoresis on 1.2% agarose gel, and the density of each band was analyzed on the Gel Image Analysis System (Tanon 2500R, Shanghai China). The levels of caspase 3- and caspase 9-mRNA were determined by calculating the density ratio of caspase 3 mRNA/β-actin mRNA or caspase 9 mRNA/ β-actin mRNA.
| Name | Upstream primer | Downstream primer | Product size (bp) |
|---|---|---|---|
| caspase 3 | 5’-ctcggtctggtacactatgtcgatg -3’ | 5’-ggttaacccgggtaagaatgtgca-3’ | 284 bp |
| caspase 9 | 5’-atggacgaagcggatcggcggctcc -3’ | 5’-ctatcctgttctcttggagagtcc-3’ | 330 bp |
| β-actin | 5’-atcacccacactgtgcccatc-3’ | 5’-acagagtacttgcgctcagga-3’ | 542 bp |
Table 1. The sequence of caspase 3, caspase 9 and β-actin.
Statistical analysis
All data were expressed as means ± standard error. Data among groups were analyzed by one-way analysis of variance (ANOVA) using the Tukey’s test to adjust for multiple comparisons or student’s t test where appropriate using SPSS 13.0 software. A value P<0.05 was considered statistically significant.
Results
Immunohistochemical analysis of caspase 3
The results of immunohistochemical of caspase 3 are illustrated in Figures 1A-1E. Caspase 3 was widely distributed throughout the mPFC region, mainly in the cytoplasm, appeared as buffy particle. The analysis results indicated that, the expressions of caspase 3 in SPS groups were significantly increased, compared with normal control group (P<0.05), and peaked at 7 day after exposure to SPS (Figure 1F).
Capase-9 immunofluorescence results
The immunofluorescence staining results of capase-9 was shown in Figure 2. The caspase-9 protein was located in cytoplasm (Figures 2A-2E). In normal control group, the fluorescent intensity of caspase-9 positive cells was poor, and that of SPS groups was significantly stronger than normal control group (P<0.01), and peaked at 4 day after exposure to SPS (Figure 2F).
SPS triggered increases in the mRNA levels of caspase-3 and caspase-9
The levels of caspase-3 and caspase-9 mRNA were normalized with β-actin mRNA level. The representative gel pattern of caspase-3, caspase-9 and β-actin cDNA bands was shown in Figure 3A. After SPS stimulation, caspase-3 and caspase-9 mRNA levels gradually increased, compared with control group, and peaked with 7 and 4 days of treatment, respectively (P<0.01) (Figure 3B). This result was consistent with that of immunohistochemical analysis.
Discussion
PTSD is thought to involve a disfunction in response to fearrelated stimuli. Four major types of characteristic symptoms of PTSD are: re-experiencing, avoidance, numbing and hyperarousal [1]. Many lines of evidence have implicated the special role of amygdala from both animal and human investigations in the processing of threat-related stimuli, in particular anger and fear is well documented [4,19]. Although many research have been made over the past decade in understanding on the molecular mechanism of PTSD, the exact pathogenesis remains obscured.
Previous studies showed the activity of amygdala in patients with PTSD increased in brain imaging. The mPFC could store memories of previous experience and play a key role in the regression on fear conditioning as well as it can inhibit amygdala activity [20]. Studies also showed that decline of mental function in PTSD symptoms in different degrees may be the mPFC lead to descending inhibition of the amygdala's inadequate. It was established that the volume of mPFC in PTSD patients decreased in the research data [21,22]. Dysfunction of mPFC is related to its reduction in volume. In this paper, we focused on this structure in order to understand further the pathological mechanism of PTSD. To clarify whether apoptosis resulted in smaller volume of mPFC, we have detected the expression of apoptosis-related proteins in the mPFC neurons of rats with PTSD in this paper.
Apoptosis is a genetically programmed, morphologically distinct form of cell death that can be triggered by a variety of physiological and pathological stimuli. It is well known that caspases, a family of cysteine-dependent aspartate-directed proteases, play critical roles in initiation and execution of apoptosis by cleaving a large number of proteins, which in turn lead to the typical morphology of apoptosis [5,23-25]. In the pathogenesis of apoptosis, the activation of caspases is a key link, which eventually led to the degradation of chromosomal DNA and cell disintegration. Caspase-9 is upstream of the start Caspase, mainly in the endogenous activation of the apoptotic pathway to activate the effect of Caspase-3 leading to apoptosis.
SPS is a model for PTSD employed extensively. SPS could also mimic the specific neuroendocrinological abnormalities observed in PTSD patients, by conducting in three stages: restraint for 2 h, forced swim for 20 min, and ether anesthesia, which correspond to psychological, physiological, and endocrinological stress, respectively. From Figure 3, we found that caspase-3 and caspase-9 mRNA levels gradually increased, compared with control group, and peaked with 7 and 4 days of treatment with SPS. The possible reason was that SPS induce the activation of caspases, which result in upregulation of caspase 9 and caspase 3 and led to dysfunction of mitochondria, which facilitated apoptotic process. Several evidences now exist to suggest that mitochondrial cytochrome release is an important control point in caspase activation and apoptosis. Li et al. showed that released cytochrome activates caspase-9 in concert with the cytoplasmic factors dATP and Apaf-1, and as a result, it consequently activates caspase-3. In this study, detection on the expression of caspase 3 and caspase 9 in mPFC neurons using immunohistochemistry, immunofluorescence and RT-PCR shows that both upregulated after SPS stimulation, which provide an experimental basis for apoptosis. All these results revealed that SPS model in this study was successfully copied, which had provided guarantee for the studies. It is also established that caspase-9 and caspase-3 play a critical role in apoptosis. At present, the pathogenesis of PTSD is not yet entirely clear. PTSD may cause a series of biochemical abnormalities and dysfunction of the mPFC, which leads to dysfunctions of brain. Thus, the pathogenesis of PTSD needs to be further studied.
The limitation of the current study is that we did not examine the contraction of mPFC and other nuclei in order to better explore the mechanism of PTSD, particularly the lack of emotion-related behavioral response in response to SPS stimuli.
Conclusions
In conclusion, these results reveal that caspase-9 and caspase-3 may play an important role in the pathology of PTSD, which provides experimental evidence for the pathogenesis of PTSD.
Acknowledgements
The authors thank all the staff members in the China Medical University Experiment Center for their technical support on how to improve the quality of the paper. In addition, this research has been supported by a grant from the National Natural Science Foundation of China (No. 81171282).
References
- Yehuda R. Post-traumatic stress disorder. New Engl J Med 2002; 346: 108-114.
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769-787.
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. DSM-IV. American Psychiatric Press Washington DC 1994.
- Ward MP, Peters KR, Smith CT. Effect of emotional and neutral declarative memory consolidation on sleep architecture. Exp Brain Res 2014; 232: 1525-1534.
- Cryns V, Yuan J. Proteases to die for. Genes Dev 1998; 12: 1551-1570.
- Cui H, Sakamoto H, Higashi S, Kawata M. Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience 2008; 152: 703-712.
- de Kloet ER. Stress: a neurobiological perspective. Tijdschr Psychiatr 2009; 51: 541-550.
- Vermetten E. Stress, trauma, and post-traumatic stress disorder. Tijdschr Psychiatr 2009; 51: 595-602.
- LeDoux JE. Emotion: clues from the brain. Ann Rev Psychol 1995; 46: 209-235.
- Ogawa S, Cohen J, Hwang D, Uchida N, Watabe-Uchida M. Organization of Monosynaptic Inputs to the Serotonin and Dopamine Neuromodulatory Systems. Cell Rep 2014; 8: 1105-1118.
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1996; 71: 55-75.
- Shin LM, Liberzon I. The Neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 2009; 35: 169-191.
- Iwamoto Y, Morinobu S, Takahashi T, Yamawaki S. Single prolonged stress increases contextual freezing and the expression of glycine transporter 1 and vesicle-associated membrane protein 2 mRNA in the hippocampus of rats. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 642-651.
- Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacol oy (Berl) 2004; 172: 225-229.
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology (Berl) 2006; 189: 165-173.
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 2003; 18: 2357-2364.
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci 2006; 26: 12967-12976.
- Paxinos G, Watson C, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 1980; 3: 129-149.
- McGauqh JL. The amygdala modulates the consolidation of memories of emotionally arousing experience. Annu Rev Neurosci 2004; 27: 1-28.
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897-905.
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002; 52: 1066-1078.
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52: 1089-1101.
- Palai TK, Mishra SR. Caspases: an apoptosis mediator. J Adv Vet Anim Res 2015; 2: 18-22.
- Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014; 5: 737-749.
- Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J Clin Invest 2015; 125: 487-489.