ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2006) Volume 17, Issue 2
Studies on tissue distribution of M1 sperm antigen using sperm-specific monoclonal antibody
1School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
2Department of Molecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2UH, UK
3Department of Obstetrics and Gynaecology, University of Sheffield, Sheffield S10 2UH, UK
- *Corresponding Author:
- Mahanem Mat
Noor School of Biosciences and Biotechnology
Faculty of Science and Technology
Universiti Kebangsaan Malaysia, 43600 Bangi
Selangor, Malaysia
Tel: 0060-3-89215193
E-mail:: mahanem@pkrisc.cc.ukm.my
Accepted date: May 17, 2006
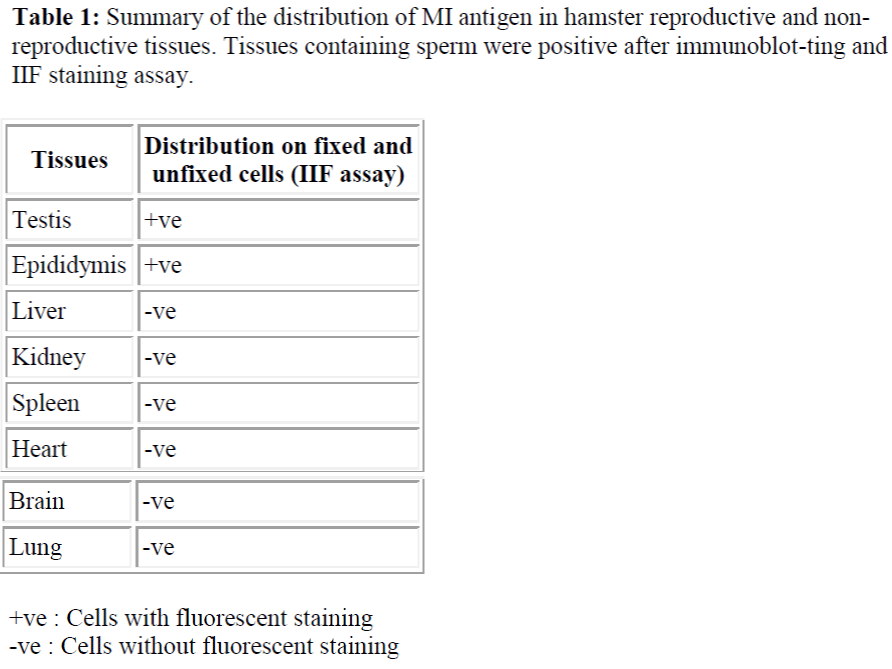
It was reported that M1 monoclonal antibody (M1 mab) inhibited hamster sperm-egg fusion in a dose-dependent manner. The antigen was localized to the equatorial segment (ES) of the hamster sperm, and that after the acrosome reaction, it was exposed on the surface of the sperm plasma membrane overlying the ES. The M1 antigen therefore fulfilled the main requirements of a sperm component that might be involved in gamete fusion processes. Hence, it was necessary to fully characterized this sperm determinant in order to elucidate its role in fertilization and to assess its potential as contraceptive antigen. In this study, M1 mab was used to analyze the developmental expression of M1 antigen during spermatogenesis and during sperm maturation in the epididymal tract. Indirect immunofluorescent staining (IIF) assay revealed that the M1 antigen was detected only in the cross sections of seminiferous tubules containing spermatids and sperm. The specificity of M1 mab was examined also by IIF assay of cell suspensions prepared from various tissues of the hamster. The result showed that the M1 mab did not cross react with cells isolated from somatic tissues including lung, heart, kidney, spleen, brain and liver. Further detection by im-munoblotting also showed that the M1 antigen appeared to be sperm-specific as it was not detected in a range of somatic cells. It is therefore proposed that the M1 an-tigen which is synthesized only by the germ cells of the testis, also found to be ex-pressed at late spermatid stage and is a sperm-specific protein.
Keywords
Tissue distribution, M1 antigen, Monoclonal antibody
Introduction
Mammalian spermatogenesis and sperm maturation are highly programmed processes during which numerous components are synthesized and degraded to fulfill the structural, functional and developmental requirements of the sperm. To analyze these complicated developmental processes, it is helpful to have antibodies which react spe-cifically with different antigenic components of spermatozoa [1]. Monoclonal antibodies have been particularly useful in this respect [2]. Information about the initial expression of sperm antigen can be useful in ascertaining sperm function. Generally, antigens expressed at or after meiosis are important for the development and function of the mature sperm rather than for maintaining the general survival of the germ cell [3,4]. Moreover, germ cell specific components are more likely to be expressed after meiosis [5].
Generally, the expression of membrane antigenic determinants of a cell can be derived either from the synthesis of novel components followed by their translocation to the membrane (possibly by vesicles) or from the modifi-cation of existing membrane components of the cell by post-translation processes such as glycosylation. This pro-cess usually occurs during spermatogenesis but additional changes may also be initiated during epididymal maturation as sperm are transported along the epididymis [4]. During this stage of sperm development many new com-ponents have been identified with monoclonal antibodies some of which originate from the epididymal epithelium. For this reason it is important to investigate sperm antigen expression in both the testis and epididymis.
As reported earlier M1 mab recognises the antigen specifically on the ES of hamster sperm but it is unknown whether this component is present elsewhere in the body. An essential requirement for a candidate antigen for a contraceptive vaccine is gamete (sperm) specificity [7]. The presence of antigen in other tissue could lead to un-desirable side effects due to antibodies mounting an auto-immune response [8]. Thus, an important aspect of anti-gen characterisation is to establish its distribution in vari-ous tissues. When the antigen is unknown but a specific antibody has been generated then immunofluorescent and immunoblotting techniques are normally performed [9].
In the present study the nature of appearance of M1 sperm antigen was investigated by IIF assay of the testicular and epididymal cells and/or in the frozen sections as well as selected somatic tissues (lung, heart, kidney, spleen, brain and liver) using M1 mab. For further confirmation, sod-ium lauryl sulphate polyacrylamide gel electrophoresis ((SDS PAGE) in conjunction with immunoblotting tech-nique was also performed.
Materials and Methods
Reagents
The M1 mab was produced at the Department of Molecular Biology and Biotechnology, University of Sheffield, UK [10]. All chemicals were purchased from Sigma Chemicals Ltd (Poole) unless otherwise stated.
Preparation of tissues for IIF and immunocytochemistry
Testes from mature hamster were decapsulated to isolate spermatogenic cells following a modified procedure of Lee and Wong [11]. Seminiferous tubules free from interstitial tissue were obtained by incubation in 0.1% colla-genase and 0.2% hyaluronidase in phosphate buffered saline (PBS) containing 0.1% glucose (PBS-G) at 37°C for 30 minutes with constant shaking. Following incubation, the cell suspension was gently pipetted and filtered through nylon screen mesh. After three washes with PBS by centrifugation at 200 g for 10 minutes, the resulting cells were resuspended in PBS-G and subjected to IIF assay. Cell suspension from epididymis was also prepared according to the same method as above.
Cell suspensions of other hamster tissues (non-reproductive organ) were prepared by passing the tissue homogenates through a muslin filter. No specific enzymatic treatment was performed. After three washes with PBS by centrifugation at 200 g for 10 minutes the cells were resuspended in PBS at pH 7.2, 4°C and subjected to IIF as-say.
To prepare frozen testis and epididymal sections, both the organs were removed from the sacrificed hamster and frozen immediately in liquid nitrogen. Subsequently, they were placed in an embedding solution (Tissue Tek II O.C.T Compound No. 4583; Miles Scientific, Naperville, IL) at 4°C and then plunged into liquid nitrogen for freezing. Sections of 10 μm thickness were cut with a cryo-tome. Tissue sections were placed on gelatin-coated glass slides, air-dried and fixed in 100% methanol prior to use for IIF, or they were stored at -20°C for later use.
M1 localization in hamster tissues by indirect im-munofluorescence staining (IIF)
Cell suspensions from each tissue sample was subjected to two different IIF assays. Cells were either methanol-fixed on slides or were maintained as a cell suspension in microcentrifuge tube (unfixed cells). Briefly for the methanol-fixed procedure, cell suspensions were smeared and left to air-dry overnight before fixation in 100% methanol. Dried slides were subjected to IIF staining as describe by Ellis et al [6].
IIF staining of various cell suspensions was the same as described for live hamster sperm. Briefly, 20 μl of cell suspension in a microcentrifugation tube was incubated with 200 μl of M1 hybridoma supernatant (undiluted) or myeloma culture supernatant (negative control) overnight at 4°C. Following this incubation, each cell suspension was centrifuged at 200 g for 10 minutes and washed three times with PBS-BSA (0.1% BSA). Secondary antibody (FITC-labeled anti-mouse IgG) at 1:100 dilution in PBS was then added for a further 2 hours incubation at 37°C. Following three centrifugation washes with PBS, the cells were transferred to glass slides for observation under the epifluorescent microscope. The percentage of stained cells was recorded for each study.
The IIF staining of the frozen testicular and epididymal sections on slide was similar to that described for methanol-fixed spermatozoa. Culture media from myeloma cells and PBS were served as negative controls.
Tissue distribution of M1 antigen by immunoblotting.
Immunoblotting was carried out using eight different tissues of the hamster; testis, epididymis, lung, heart, kidney, spleen, brain and liver. The tissues were removed immediately after the animals were sacrificed and frozen in liquid nitrogen. Subsequently they were homogenized in PBS containing 2 mM phenylmethanesulfonylfluoride (PMSF), a protease inhibitor. Cellular pellets were solubi-lised in 10 volumes of 0.5% NP 40, precipitated in acetone and solubilised in 50 mM Tris-HCl buffer containing 2% SDS and 5% β mercaptoethanol. Ten microgram of each of the protein samples was submitted to SDS-PAGE and transferred to a nitrocellulose membrane. Blotted membranes were then incubated with undiluted M1 hy-bridoma supernatant and processed as described previ-ously [10].
Protein determination
The amount of protein in the sperm and in the tissue extractions were determined using a bicinchoninic acid protein assay kit (Pierce Chemical, Rockford,IL) according to the supplier instructions.
Results
Indirect immunofluorescence and immunohistochemical staining
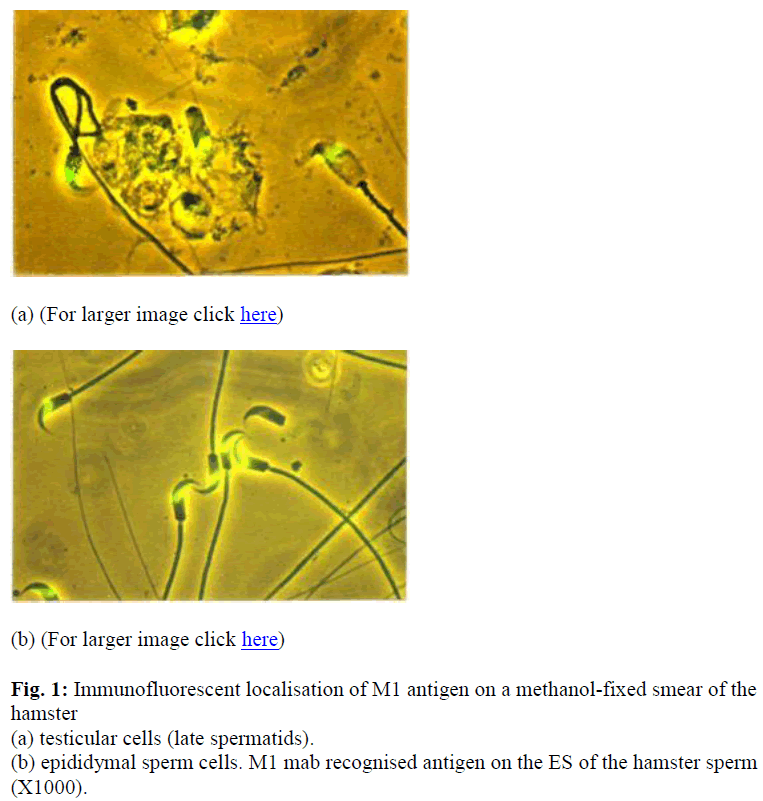
The specificity of M1 antibody was examined by IIF of fixed and unfixed cells that were prepared from various tissues of the hamster. As summarised in table 1.0, M1 antibody did not cross-react with cells isolated from somatic tissues including lung, heart, kidney, spleen, brain and liver but was spermspecific (see Table 1 & Figure 1). Additionally, immunohistochemical staining analysis of frozen sections of hamster testis and epididymis confirmed that the M1 antigen was only expressed on sperm cells at the very late spermatid stage. As shown in Figure 2 (a) M1 antigen was detected only on cross sections of testes seminiferous tubules containing spermatids and immature sperm.
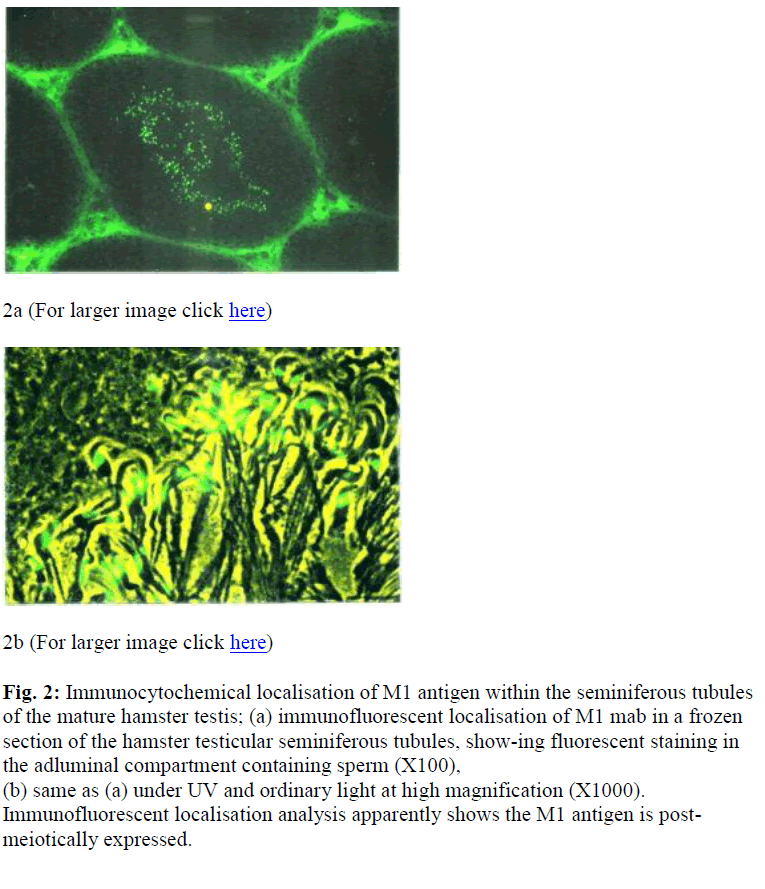
Figure 2: Immunocytochemical localisation of M1 antigen within the seminiferous tubules of the mature hamster testis; (a) immunofluorescent localisation of M1 mab in a frozen section of the hamster testicular seminiferous tubules, show-ing fluorescent staining in the adluminal compartment containing sperm (X100), (b) same as (a) under UV and ordinary light at high magnification (X1000). Immunofluorescent localisation analysis apparently shows the M1 antigen is post-meiotically expressed.
At a higher magnification, the fluorescent stain was local-ised to the ES of developing spermatids [Figure 2 (b)]. Control sections displayed some staining in the interstitial tissue and it was considered to be due to non-specific binding (Moore, personal communication). The formation of the ES could be followed by examining germ cells at various stages of development.
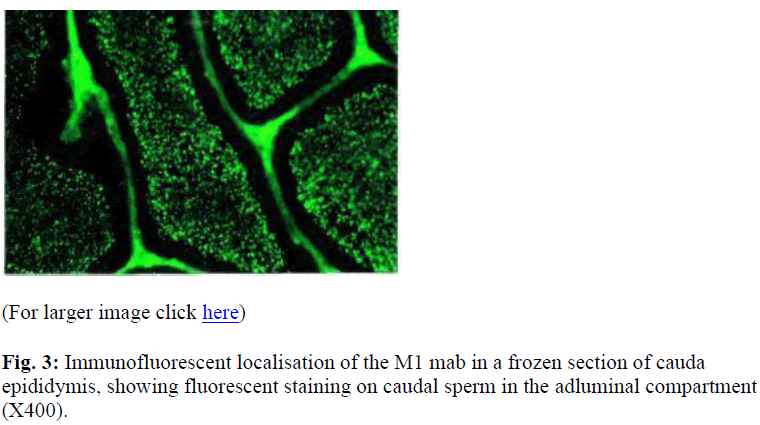
Intensed staining was exhibited by the late spermatid stage. On sections of epididymis, intensed staining was seen in the epididymal lumen in both the caput (data not shown) and cauda (Figure 3). There was no apparent change in the intensity of staining on the sperm in the epididymis.
Immunoblotting for M1 antigen in the testes, epididymis and other tissues
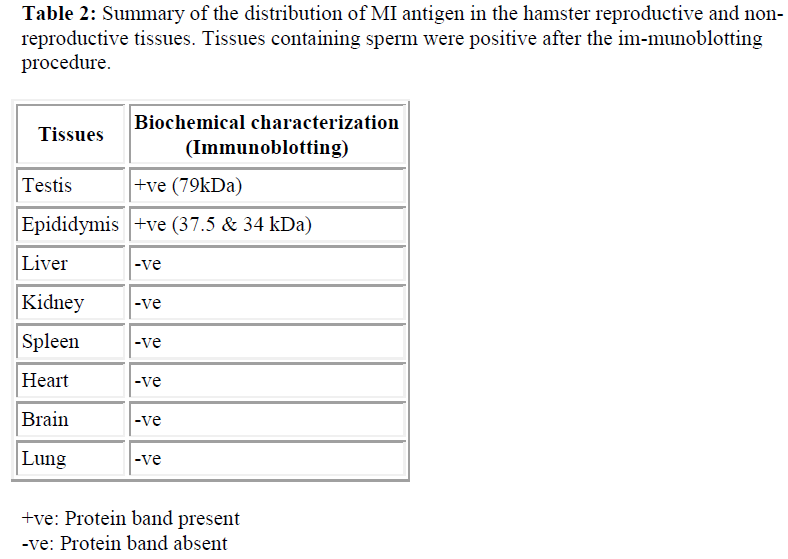
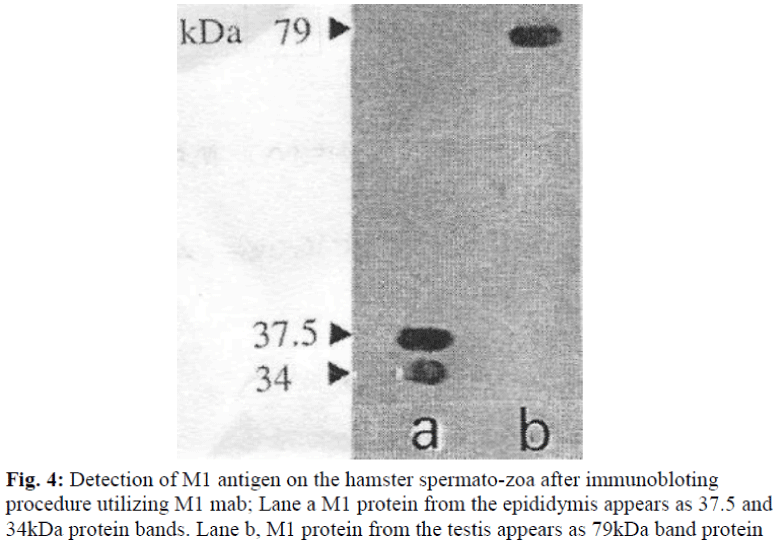
Since SDS-PAGE analysis only represents the pattern of total protein present in the sample, immunoblotting was undertaken to detect whether a protein was recognized by M1 antibody. The antibody was used to probe immunoblasts of SDS-PAGE gels of protein samples obtained from the the hamster; testis and epididymis, and other tissue, such as lung, heart, kidney, spleen, brain and liver. The M1 mab only reacted against the testis (79kDa) and epididymis (37.5 and 34 kDa) (see Table 2 and Figure 4).
For all other samples no immunoreactivity was detected at any level of the molecular weight. Control immunoblots incubated with myeloma medium showed no immu-noreactivity at all (data not shown).
Discussion
IIF of cells released from the testis demonstrated that M1 antigen was only present in the developing (post-meiotic) or mature testicular sperm. The absence of M1 antigen in other testicular cells indicated that it was not secreted by Sertoli cells for example and taken up by the germ cells. Since the preparations were smears of the cell suspension, the developmental progress of M1 antigen during spermatogenesis could not be seen. Therefore, a more detailed immunohistochemical study with frozen sections of the hamster testis and epididymis was undertaken.
Sperm cells have unusual membranes compared to those of the somatic cells. As discussed by Phelps et al [12], the initial organisation of the sperm surface into discrete membrane domains begins during spermiogenesis and continues as the sperm cells traverse through the epidi-dymal duct. A delicate reorientation and modification of plasma membrane molecules then take place in the female tract before the fertilization occurs [13]. Previous studies also suggest that antigens appear on the sperm at different times from the beginning of spermiogenesis. In guinea-pigs for example, PH20 first appears at step 6-7 of the spermiogenesis [14] while PH30 antigen first appears on the sperm in the cauda epididymis [15]. However, im-munofluorescence localisation on the fixed cryosections of the hamster testis indicated that M1 antigen was first expressed during postmeiotic differentiation at elongating spermatid, concomitant with the development of the apical acrosomal and equatorial region (Figure 1a). The M1 antigen was well established when sperm entered the epididymis. There was no fluorescent staining on epididymal epithelial cells. Although the connective tissue surrounding the epididymal tubules exhibited staining, this was also seen in controls and thus represented nonspecific binding as also seen in the testis. Therefore, it can be concluded that the M1 antigen is synthesised only by germ cells in the male reproductive tissue.
Immunoblot analysis with protein extracts from the testis and epididymis sperm revealed different molecular weight was detected by M1 antibody. Two main bands were de-tected at 37.5 and 34 kDa for epididymal sperm samples and 79kDa for testicular sperm samples, suggesting that M1 antigen is initially present as a protein and then under certain proteolytic condition it splits into two subunits 37.5 and 34 kDa. Multiple forms of peptides that are iden-tified by immunoblotting may be the consequence of (i) post-translational modification, (ii) proteolytic processing of the proteins within the acrosome, (iii) multiple gene product, or several of these possibilities acting in concert.
The likelihood of sperm antigen undergoing proteolytic cleavage during maturation is quite high. For example cleavage of PH-30 antigen occurs while the sperm are in the epididymis. This may be caused either by epididymal secretions [4] or due to some dissociated sperm acrosome proteases that affect intact cells. Because the population of acrosome-intact sperm, immediately after removal from the male guinea-pig normally contain about 10-15% cells without acrosomes [16].
The molecular profile of M1 antigen exhibited some simi-larity with the mouse sperm antigen (40 kDa) detected by M29 mab [17] and also the guinea-pig sperm antigen (34 kDa) [18]. Both these antigens are localised to the ES of the sperm. Although M1 antigen displays the same molecular weight as DE protein [19], it is reported to be involved in the rat sperm-egg fusion [20], and it is unlikely to be the same molecule. The reason for this is that M1 antigen is produced by the sperm during the late spermatid stage while DE is a sperm-coating protein secreted by the epididymis. Furthermore, M1 antibody does not cross-react with the rat sperm. It has been reported by Calvete et al [21], that horse sperm plasma 3 (HSP-3) is involved in sperm-egg fusion since it possesses a high amino-acid sequence similarity with the DE protein [19]. Calvete and his colleagues [21] moreover demonstrated a 16 kDa stallion spermadhesin which is present in another membrane fraction (HSP-7), localised on the ES of the ejaculated stallion sperm and it may possibly be a putative sperm-egg fusion protein. Again, based on the present result M1 antigen is found not to be similar to HSP-3 and HSP-7 proteins even though they are structurally con-served in several mammalian species.
Based on IIF and immunoblotting results, M1 antigen appeared to be sperm-specific as it was not detected in a range of somatic cells. The result is entirely in agreement with the proposed function in relation to fertilization, since gamete interactions are thought to be tissue specific [22].
In conclusion, the results to date suggest that M1 antigen is sperm specific, synthesized only by the sperm cells and is located in the crypt of the ES. The M1 mab against this protein was proven to be involved in sperm-egg fusion by reducing fertilization rate in vitro [10] and in vivo without any detectable side effects [23]. This protein is, therefore, likely to be important for the development of a potential and safe candidate for an immunocontraceptive vaccine. Investigations at the molecular level are essential and proteomic of M1 antigen is in progress.
Acknowledgements
The editorial assistance of Professor Dr. Mohd Hamim Rajikin is gratefully acknowledged. This work was supported by research grant from Ministry of Science and Technology Malaysia.
References
- Bellve AR, Moss R. Monoclonal antibodies as a probe of reproductive mechanism. Biol Reprod 1983; 28:1
- Eddy EM, O’Brien DA. The spermatozoon. In: The Physiology Of Reproduction. Knobil E, Neil JD (Eds.) Raven Press, New York.1994; 1: 29-77.
- Sharpe RM. Regulation of spermatogenesis In: The Physiology Of Reproduction. Second edition. Knobil E, Neil JD (Eds.) Raven Press. New York.1994; 1: 1363-1434
- Moore HDM. Sperm maturation and transport in the excurrent ducts. In: Gametes-The Spermatozoon. Gruzinskas JG, Yovich, J (Eds.) Cambridge University Press, Cambridge. 1995;140-156
- Moore HDM, Bedford JM. The interaction of mammalian gametes in the female. In: Mechanism And Control Of Animal Fertilization. Hartman JF (Eds.) Academic Press, New York. 1983; 453-497
- Ellis DH, Hartman TD, Moore HDM. Maturation and function of the hamster spermatozoon probed with monoclonal antibodies. J Reprod Immunol 1985; 7: 299-314.
- Aitken RJ. Immunocontraceptive vaccines for human use. J Reprod Immunology 2002; 57: 273-287
- Alexander NJ, Bialy G. Contraceptive Vaccine Development. Reprod Fertil Dev 1994; 6: 273-280.
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbour Laboratory, New York 1988.
- Mat Noor M, Moore HDM. Monoclonal antibody that recognizes an epitope of the sperm equatorial region and specifically inhibits sperm-oolemma fusion but not binding. J Reprod Fert 1999; 115: 215-224
- Lee CYG, Wong E. Developmental studies of sperm surface antigens using sperm-specific monoclonal antibodies. J Reprod Immunol 1986; 9: 275-287
- Phelps BM, Koppel DE, Primakoff P, Myles DG. Evidence that proteolysis of the surface is an initial steps in the mechanism of formation of sperm cell surface do-mains. J Cell Biol 1990; 111: 1839-1847
- Flesch F M, Gadella M. Dynamic of the mammalian sperm membrane in the process of fertilization. Biochemica Biophysica Acta 2000; 1469: 197-235
- Cowan AE, Myles DG, Koppel DE. Lateral diffusion of the PH 20 protein on guinea pig sperm: Evidence that barriers to diffusion maintain plasma membrane do-mains in mammalian sperm. J Cell Biol 1987; 104 : 917-923
- Primakoff P, Hyatt H, Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol 1987; 104 : 141-149
- Primakoff P, Cowan A, Hyatt H, Tredick-Kline J, Myles DG. Purification of the guinea pig sperm PH 20 antigen and detection of a site-specific endoproteolytic activity in sperm preparations that cleaves PH 20 into two disulfide-linked fragments. Biol Reprod 1988; 38 : 921-934
- Saling PM, Irons G, Waibel R. Mouse sperm antigens that participate in fertilization.I. Inhibition of sperm fusion with the egg plasma membrane using monoclonal antibodies. Biol Reprod 1985; 33: 515-526
- Allen CA, Green DPL. Monoclonal antibodies which recognize equatorial segment epitopes presented de novo following the A23187-induced acrosome reaction of guinea pig sperm. J Cell Science 1995; 108 : 767-777
- Brooks DE, Means AR, Wright EJ, Singh SP, Tiver KK. Molecular cloning of the cDNA for androgen-dependent sperm-coating glycoproteins secreted by rat epididymis. Eur J Biochem 1986; 161:13-18
- Rochwerger L, Cohen DJ, Cuasnicu PS. Mammalian sperm-egg fusion: The rat egg has complementary sites for a sperm protein that mediates gamete fusion. Dev Biol 1992 ; 153: 83-90
- Calvete JJ, Nessau S, Mann K, Sanz L, Sieme H, Klug E, Topfer-Petersen E. Isolation and biochemical characterisation of stallion seminal-plasma proteins. Reprod Dom Anim 1994; 29 : 411-426
- Yanagimachi R. Mammalian fertilization. In: The Physiology of Reproduction. Second edition. Knobil E, Neil JD (Eds.) Raven Press. New York 1994;1: 189-317
- Mahanem MN, Dzulsuhaimi D. The effect of M1 mono-clonal antibody on in vivo fertilization in hamsters. Mal Appl Biol 2003; 32: 15-20