ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 1
Study of related factors about positive biological monitoring of steam sterilization and emergent treatment
Central Sterile Supply Center of West China Hospital at Sichuan University, No 37, Guoxue Road, Chengdu City, Sichuan Province, PR China
- *Corresponding Author:
- Zhou Xiao Li
Central Sterile Supply Center of West China Hospital at Sichuan University
Sichuan Province, PR China
Accepted date: October 24, 2017
DOI: 10.4066/biomedicalresearch.29-17-903
Visit for more related articles at Biomedical ResearchObjective: This study aimed to gain relevant experience from an emergent treatment about steam sterilization rapid biological monitoring (BI).
Methods: To analyse the positive result of BI of steam sterilization and take effective measures, including stopping distributing, recalling all items processed back to the last negative BI, analyzing the physical and chemical monitoring back to the last negative BI, and repairing sterilizer.
Results: Recall 36 surgical instrument packages, use other sterilization method, and distribute the items after BI. No surgery was disturbed. The reason that Belimed MST9-6-18HS2 prevacuum steam sterilizer failed was aging of rubber gasket inside pressure switch, which led to leak. After replacing the sterilizer pressure switches and accessories, BI and Bowie-Dick tests were passed for three times and sterilizer was reused.
Conclusion: Except for the conventional maintenance, the sterilizer needed regular and deep inspection and timely replacement of wearing or related accessories. Effective emergent treatment should be taken to control the medical risks produced by positive BI.
Keywords
Biological monitoring, Positive reports, Emergent treatment, Related research.
Introduction
Biological monitoring (BI) is the gold standard using nonpathogenic bacteria spores to determine and evaluate the effect of steam sterilization [1]. The use of spores based standard BI has been accepted practice for establishing that a sterilization process has been effective in sterilizing a given batch of surgical instruments in acute care facilities and medical device manufacturing for over 30 years [2]. If these spores are destroyed, it is assumed that any contaminating organisms in the load have also been killed, as these organisms have lower resistance than the spores, and are present in lower numbers [3]. The traditional biological indicators were inoculated paper strips inside envelopes, which were transferred to sterile culture medium following processing, and incubated for 7 d [4]. Recent years, rapid BI is widely used to get the result quickly and accurately, which shorten the detection time greatly [5]. From Zhang et al. they have compared the accuracy of the sterilization BI effect of three kinds of pressure steam sterilization integrating indicators by real surveillance [6]. Another study also reported the monitor of sterilization effect of different kinds of chemical indicator for steam sterilization [7]. All the surgical instruments will not be distributed until BI was negative, which makes the sterilization process safe. In the Central Sterile Supply Department of West China Hospital at Sichuan University, one positive case of BI in steam sterilization was reported in January 1, 2014. It was timely analysed and effective measures were taken to make sure the safe use.
Materials and Methods
Materials
Belimed MST9-6-18HS2 prevacuum steam sterilizer was used. The sterilization temperature was set to 134°C, sterilization time was set to 6 min. 3 M 41382 integrating Process Challenge Device (PCD) containing 3 M 1292 biological indicator and class 5 integrating indicator was placed in the area of the sterilizer chamber when the sterilization cycle began. The PCD was placed on the bottom section of the sterilizer drain near the gate. The item name, the end user of the items and the sterilizer number was written on record sheet outside the PCD. The BI was performed by 3 M 290 auto reader after physical monitoring and chemical monitoring of the sterilization cycle was passed.
Biological monitoring operating
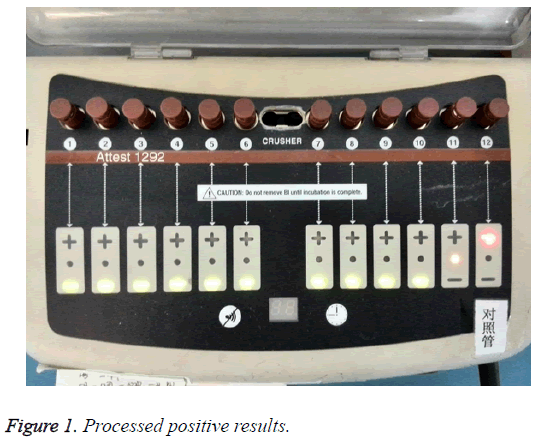
The 3 M 41382 integrated PCD was used in accordance with introduction for users. Each information of biological indicator in the reading hole, such as pot number and type, was recorded. Positive control from same lot number as processed biological indicators at the same day was put in incubation well for incubating in 1 h. The BI result was obtained after 3 h. The yellow light indicating the incubation was ongoing. The green light indicating the negative result was detected, and the red light indicating the positive result was detected (Figure 1). No bacteria will grow if the media in the processed BI tube remained purple, which provided negative result. The culture media in positive control tube turned yellow and cloudy that indicated a growth of bacteria and positive. Processed BI negative result with positive control indicated acceptable sterilization process (Figure 2) [8].
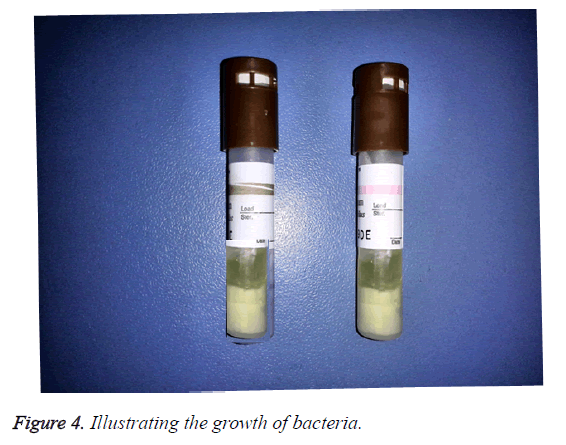
Positive results: In the morning, the nurse reported a positive case. Two red (+) symbol was displayed on the LCD panel of auto reader. One biological indicator was processed, another was positive control. The other lights displayed green-symbol, which indicated negative result (Figure 3). The culture media in the processed indicator and positive control were yellow and cloudy, which illustrated the growth of bacteria (Figure 4). The media color of rest processed indicators were purple, which meant no bacterial growth. The color of chemical indicator outside the tube was brown, which meant the BIs were exposed to the sterilization process. The color of chemical indicator outside the tube was pink, which meant the BIs were not exposed to the sterilization process. In conclusion, positive BI case was reported.
Emergency measures-stop distributing
All processed items distributing were stopped immediately. Nurse on duty reported to supervisor followed recall procedure. This batch items were reprocessed by other sterilizer or sterilization method in order to ensure the supply of surgical instruments and clinical items.
Recall and reprocess
According to “Central sterile supply department (CSSD) Part III: surveillance standard for cleaning, disinfection and sterilization”, all the unused sterile items should be recalled as soon as possible back to the last negative BI. The items should be reprocessed and the causes should be analysed for improvement. The sterilizer can be reused after three consecutive BI [9]. According to this protocol, the instruments were recalled and reprocessed. Other autoclave sterilizers were used to process the items.
Suspend of sterilize
The number of the dysfunctional sterilizer was recorded and suspended. The logo read "fault, suspended".
Rapid biological monitoring equipment performance detecting
The biological indicator processed by functional sterilizer was put in the well with positive control. No positive alarm after three hours as normal operation. Thus, the cause of BI equipment malfunction was excluded.
Sterilizer leak test
The engineer arrived within two hours. Empty chamber leak test of sterilizer was conducted after analysis. It was found that the leak was for the error of pressure switch, not the error of sterilizer door seal after leak test.
Maintenance of sterilizer
Engineers replaced the pressure switch of MST9-6-18HS2 prevacuum steam sterilizer. According to “Central sterile supply department (CSSD) Part III: surveillance standard for cleaning, disinfection and sterilization” [9], three consecutive Bowie-Dick tests were run after sterilizer maintenance service. Then physical monitoring, chemical monitoring and BI were completed with three consecutive sterilization programs. In the end, the hollow PCD tests were run for three times to make sterilizer properly qualified before put into use. The details and data of incident were saved for future reference according to relevant provisions.
Results
In the steam sterilization positive BI report, 36 surgical instrument packages were recalled wherein one implant package, which were all repackaged to sterile by using other steam sterilizers. Surgical instruments were delayed 1.5 h to use. Implants were distributed after BI passed in 4.5 h. No surgery and clinical use were affected. The rubber gasket of the pressure switch of MST9-6-18HS2 prevacuum steam sterilizer was aging to leak. The leak rate is greater than 3 mpa/min. Sterilizer was normally enabled to use after replacement of pressure switch and success of leak test with no leak.
Discussion
Principle of biological monitoring
Steam sterilization was widely used in hospital because of good effect, short time and low cost. Sterilization process conditions should be evaluated by physical, biological, and chemical monitoring. Physical monitoring reflected if the sterilization cycle was complete. Chemical monitoring had many impact factors. Therefore, BI was the most accurate and reliable method in quality control of steam sterilization [10]. Biological indicators monitored high-pressure steam sterilization under 132°C, 4min condition with resistant bacterial spores to make Sterility Assurance Level (SAL) expressed as 10-6. Biological indicator was applied to ensure the entire sterilization process can achieve at the level of SAL. It reflected a variety of factors led to sterilization failure comprehensively. 3 M rapid biological indicator contained a standardized amount of Bacillus stearothermophilus spores. The culture media of biological indicator involved water, pH indicator, nutritious substances and fluorescent substrate. Rapid BI provided a strong guarantee for the safety of surgical instruments [11].
Analysis of biological monitoring
Positive result of rapid BI could not prove sterilization failure [12]. Some factors also led to false positive results, such as no proper six-step hand-washing before the operating, chemical indicator outside the self-contained BI touched by fingers, indicator cap untimely pressed and other improper operation of biological indicators. All operations may be polluted that could provide false positive results. In this case, the validity of biological indicators, the production lot to ensure the comparability between processed and unprocessed BI (Figure 2), incubation temperature within the range of 56 ± 1°C, the cleanliness of the reading hole, the pollution in the incubation well, fail working condition caused by the unrepaired lamps which may cause the temperature detector error, foreign substance barrier leading to interpretation errors, etc. In this case, operations were followed the recommendation, biological indicators were effective and the detector had no faults after investigating. Above all, concluded no false positive results.
Analysis of sterilizer
It should be checked that the class 5 integrating indicator in integrated PCD, the chemical indicators inside and outside the package of sterilization items. The record of chemical monitoring especially Bowie-Dick test in the past week should be checked to find the reason for sterilizer malfunction. No Bowie-Dick test failure and chemical indicators meant acceptable sterilization process. The physical monitoring records should be checked to ensure the consistency between sterilization cycle program and the sterile items, the parameters of sterilization program and the alarm reminder. After that the physical monitoring should be compared with other normal qualified physical monitoring records. The evacuation times, evacuation duration, final pressure, sterilization temperature and sterilization duration should be checked in order to look for physical problems. The manufacturer engineer was contacted to discover sterilizer hardware problems by a leak test. Maximum leak rate of Belimed MST9-6-18HS2 prevacuum steam sterilizer was less than 3 mpa/min. The detection value was greater than 3 mpa/min proved the sterilizer leakage. Engineers found that pressure switch problem caused sterilizer leakage in an exact testing method. The leakage existed but the Bowie-Dick test still passed, because the leak rate was less than predetermined value of sterilizer. After Bowie-Dick test, the rubber gasket of pressure switch inside prevacuum steam sterilizer aged and leaked worse until leakage rate was greater than 3 mpa/min, which resulted in the sterilization failure. Some experience was gained through this case that steam sterilizer should be regularly checked by special and deep inspection. The timely replacement of accessories was necessary to ensure the sterilization quality.
Conclusion
The low probability of positive BI and the less experienced nurses to deal with the positive results of rapid BI led to incorrect or untimely treatment, which brought out unsafety of processed items. Except for the daily maintenance of steam sterilizer, there should be regular special and deep inspection, and the timely replacement of accessories. When the positive BI occurred, the surveillance people should pay great attention to report according to process. Effective measures should be taken to control medical risks and ensure medical safety as positive BI appeared.
References
- Qin G, Li L. Impact factors and effection of biological monitoring in steam sterilization. Chinese J Disinfect 2012; 29: 621.
- Gordon PE. P389: A rapid biological indicator for sterility assurance. Antimicrob Resist Infect Contr 2013; 2: 389.
- Pflug IJ, Odlaug TE. Biological indicators in the pharmaceutical and the medical device industry. J Parent Sci Technol Publ Parent Drug Assoc 1986; 40: 242.
- Belkin NL. Comparison of a rapid readout biological indicator for steam sterilization with four conventional biological indicators and five chemical indicators. Infect Off J Soc Hosp Epidemiolog Am 1996; 17: 773.
- Chen X. Defect analysis of rapid biological monitoring. J Med Theor Pract 2012; 25: 1905-1906.
- Zhang J, Xin-Wu LI, Zhang W. Study on the monitor of sterilization effect of differene kinds of chemical indicator for pressure steam sterilization. Chinese J Disinfect 2015; 9: 233.
- Belkin NL. Comparison of a rapid readout biological indicator for steam sterilization with four conventional biological indicators and five chemical indicators. Infect Contr Off J Soc Hosp Epidemiolog Am 1996; 17: 773.
- Yu L, Fu C. Analysis of positive results in steam sterilization biological monitoring. Chinese J Disinfect 2009; 26: 439.
- Chinese Ministry of Health. Central sterile supply department (CSSD) Part III: surveillance standard for cleaning, disinfection and sterilization. Chinese Stand Books 2009.
- Shen J, Zhang L. Progress of steam sterilizers. Chinese J Disinfect 2007; 24: 271.
- Xiao Q. Fitting of chemical monitoring and biological monitoring in steam sterilization. Chinese J Nosocomiol 2012; 22: 141.
- Chen J, Tie J, Wang C. Research of rapid biological indicators culture method consistency in steam sterilization. Chinese Prev Med 2013; 14: 831.