ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 5
Study on the active compounds of Sisymbrium irio L. grown in agricultural fields west of mosul city.
Fathi A. Al-Mandeel*
Environment Research Center, University of Mosul, Mosul, Iraq
- Corresponding Author:
- Fathi A. Al-Mandeel
Environment Research Center
University of Mosul
Mosul
Iraq
Accepted date: May 27, 2022
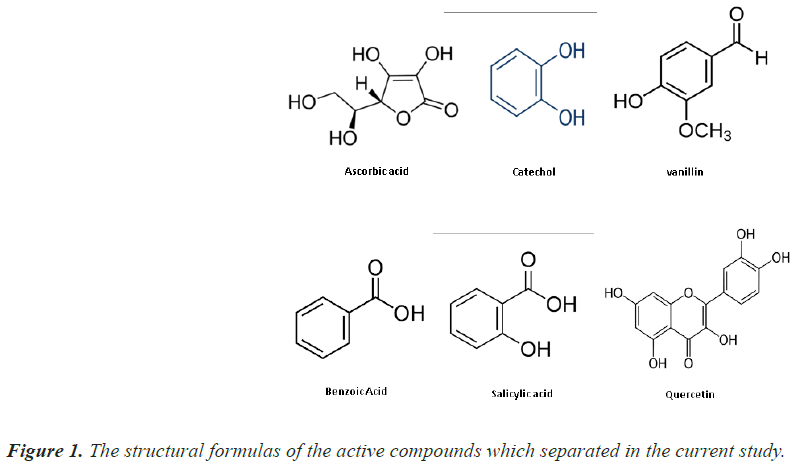
In this study, active compounds such as: of Ascorbic acid, Catechol, Vanillin, Benzoic acid, Salicylic acid and Quercetin separated from crude of Herbs belonging to the Sisymbrium irio L that collected from Al-Qawsiat area, which is located within mosul city.
Separating process of compounds was carried out through high-performance liquid chromatography technique, the samples examined by LC-20 AD ShimadZu HPLC, which included C18 (240 × 4.60 mm) column chromatography.
Quantitatively, the concentrations of the active compounds under study ranged between (0.06080 and 0.00008) mg/g of plant tissue, Ascorbic acid was the highest quantitatively, then Vanillin, Benzoic acid, Catechol, Salicylic acid, While Quercetin was at the end of the series in quantitative terms.
Environmentally, results showed that the Concentrations of the positive ions( Na1+, K1+, Ca2+ and Mg2+) were (78,15,235 and 47,97) ppm respectively, while the negative ions (Cl-1, HCO3 -1 and SO4 -2) were (68,69 and 187.43) ppm respectively, while the major nutrients (ready phosphorous and ready nitrogen) amounted to (1.08 and 42.12) ppm respectively, and these results indicate that the soil under study is very suitable for plant growth, but it must be said that Most of the positive ions under study were within the low critical limits.
Keywords
Phenolic compounds, HPLC, Sisymbrium irio, Active compounds, Environment.
Introduction
The Brassicaceae (cruciferae) the most widespread families in the plant kingdom [1], this family contains more than 350 genera and 3,000 species worldwide [2]. One of these genera is Sisymbrium irio. It is one of the most used herbs in the middle east to treat coughs, chest congestion, detoxify the liver, and clean wound [3].
The spread of phenolic compounds in different plant families encouraged researchers to investigate facts related to these compounds. For example, four chemical compounds were observed to be the potential inhibitory source against the 6COX_A protein. The active compounds of V. negundo which diagnosed with GC-MS, showed a better interaction analysis and docking simulation, than the existing COX-2 inhibitor such as ibuprofen and aspirin.
In a study conducted by Merah et al., [4], the analysis by HPLC-DAD of organic extracts from needles of theAlgerian Pinus coulteri showed presence of 10 phenolic acids and nine flavonoids. They report that P. coulteri possesses high biological activities and thus could represent an interesting alternative to natural and preventative treatments.
Also Six compounds identified as: 3,11-dihydroxy-12- oleanene, pentadecanoic acid, spinasterol, spinastero l-3-O-β-D-glucopyranoside and saikochromic acid were isolated from the extracts obtained from the aerial parts of P. verticillatum and the results of showed that the extracts have great potential antimicrobial compounds against microorganisms [5].
The antioxidant and antiproliferative effects were studied on extracts containing tamrixetine, caffeic acid and rutin isolated from B. hispanica, and the structures of the purified compounds were elucidated by spectroscopy and mass spectrometry. In addition, extracts of B. hispanica were evaluated against six bacterial strains. The results showed that the crude extracts of Brucella hispanica had remarkable antibacterial and antioxidant activity. Moreover, the results showed a strong cytotoxic effect on HeLa cells [6].
Due to their pharmacological properties as being antioxidants, there is a noticeable increase in the number of studies dealing with active compounds. These compounds are found in all parts of the plant, as the plant can build thousands of different phenolic compounds that share one or more hydroxy aromatic rings and they help the plant to change its biotic and abiotic environment, It also gives the plant color and taste, as well as protective benefits that contribute to encouraging the plant to reproduce. Plant phenols are among the most studied plant chemicals in different fields [7].
With regard to secondary metabolism, it is known that the pharmacological activities of any plant are due to the presence of active compounds in it, which include steroids, carbohydrates, phenolic compounds, saponins, glycosides, tannins, flavonoids and alkaloids [8].
Phenolic compounds are secondary metabolites formed during the natural growth of the plant [9] as well as a reaction to stress conditions such as infections, wounding, and Ultraviolet rays (UV) which the plant may be exposed to it [10]. Phenols are widely found in plants and are a very diversified group of plant chemicals derived from phenylalanine and tyrosin. They are not evenly distributed in tissues, as well as at the cellular and subcellular lavel. For example, insoluble phenols are one of the components of cell walls, While soluble phenols are present as Compartmentalized classes in plant cell vacuoles, at the level of tissues, the outer layers of the plant contain high levels of phenols compared to those that fall to the inside of it [11].
Cellual phenolic such as lignin (a polymer consisting of secondary units) as well as hydroxycinnamic acids are linked to various cell components, so these compounds contribute to the mechanical strength of cell walls as well as they play a role in morphogenesis as well as in the response of plants Stress and pathogens [12].
The major phenolic acids such as ferulic compounds as well as p-coumaric acids are major phenolic acids may esterified with pectins and arabinoxylans, also it can esterified with cross-linked to cell wall polysaccharides in the form of dimers such as dehydroferulates and truxillic acid and Truxillic acid [13], so it is suggested that these cross-linkers can be play a role in cell-to-cell adhesion [14].
In terms of their importance to humans, phenolic compounds are widely found in food, and they represent important components of the human diet (low-sugar food). They can also be protective against cardiovascular disease, in addition to having latent anti-cancer properties due to their antioxidant activity [15].
Weeds of Sisymbrium irio were collected from the soil far from human agricultural and industrial activity to be a goal for identify a number of secondary metabolites (quantitatively and qualitatively) by chromatographic techniques. So six standards of active compounds used to achieve the objectives of the current study.
Materials and Methods
TThe standards of (Ascorbic acid, Catechol, Vanillin, Benzoic acid, Salicylic acid and Quercetin) used to achieve the aims of the study by a series steps are:
Methods of extraction
Plant sample preparation: After drying the samples, extraction process was done with 95% ethanol, and ethanolic extract concentrating by using a Rotary Evaporator (RVE), then hydrolysis of the crude solution carried out with HCL (1 N) and the non-sugar phenols isolated by ethyl acetate [8].
Methods of separation: Separation process carries out in the chemical analysis lab at Al-Kindy stat company in Mosul city based on the principle that mentioned [7]. Samples examined by LC-2010 AHT ShimadZu HPLC after purification by membrane filters of 0.45 µM.
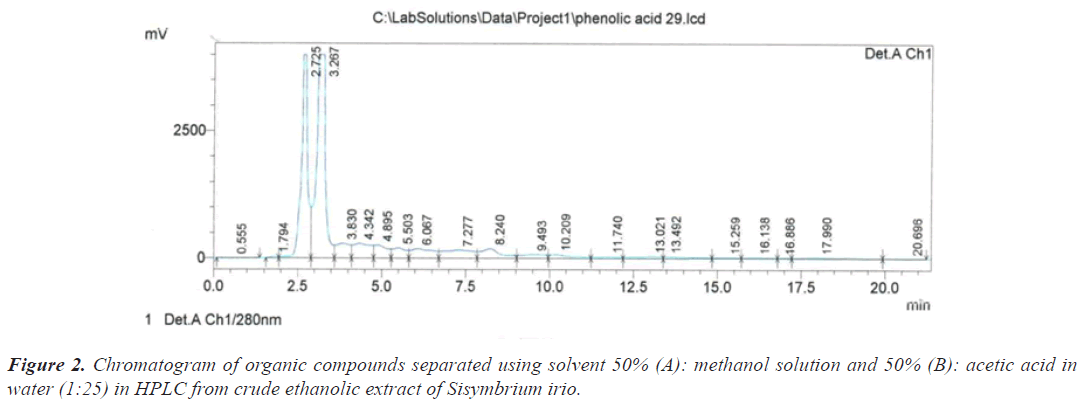
The separation was conducted using wavelength 280 nm, a flow rate of 1 mL/min and Gradient elution of two solvents (A): methanol and (B): (acetic acid in water) at a ratio (1 to 25). Separation process lasted for 25 minutes by Gradient program at 50% of solution (A) and 50% of solution (B), using a C18 (240 × 4.60 mm), and the injection process was 20 lµ.
Standard solution: Standard of (Ascorbic acid, Catechol, Vanillin, Benzoic acid, Salicylic acid, Quercetin) from the Chemistry Department of Science college in Mosul University, and they are a product of Floka Swiss company as well as BDH Co.
The standard solution was prepared by dissolving 6 mg of the compound in 10 ml of methanol to obtain 600 µg/ml of methanol, then solution filtering by (0.45 µm) filter membrane. The content of active compounds in the samples was calculated using the following equation:
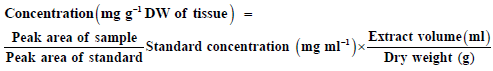
Soil analysis
Soil and vegetation samples collected from the Al-Qawsiat area, which is located in northern Iraq, within Mosul city, between (36'20'00N and 43'70'00E). Soil samples were collected at a depth of (25 cm), by hand soil auger from five locations, 10 meters apart from each other, in order for the sample to represent the whole study area. After drying, all samples were mixed, ground and sieved with a sieve with holes (2.00 mm).
After making an extract from the soil using distilled water at a ratio (1:1)., Estimation of EC, pH, as well as some of positive and negative elements such as: Na1+, K1+, Ca2+, Mg2+, Cl1-, HCO3 1- and SO4 2- carried out in the laboratories of the Mosul university, To determine the soil content of heavy metals (lead, zinc, copper and Iron), an extract made from the soil and a solution of DTPA (0,005) Muller with an extraction ratio of (1:2) with shaking for two hours and then filtered [16], and then the microelements were estimated by a device Atomic absorption type NOVA, In the chemical analysis process relied on the methods of work that he mentioned [17,18].
Results and Discussion
First: Phytochemical analysis
In this study, six compounds whose structural formulas are shown in Figure 1 separated after preparing the crude extract of the herbs belonging to the Sisymbrium irio L that collected from Al-Qawsiat area, which is located within Mosul City, between latitude 36°20’00N Longitude 70'00'°43E (Table 1).
| Samples (crud extracts) | Standard Compounds | ||
|---|---|---|---|
| Retention time (Rt) | Compounds | No | Retention time (Rt) |
| 2.736 | Ascorbic acid | 1 | 2.725 |
| 4.654 | Catechol | 2 | 4.895 |
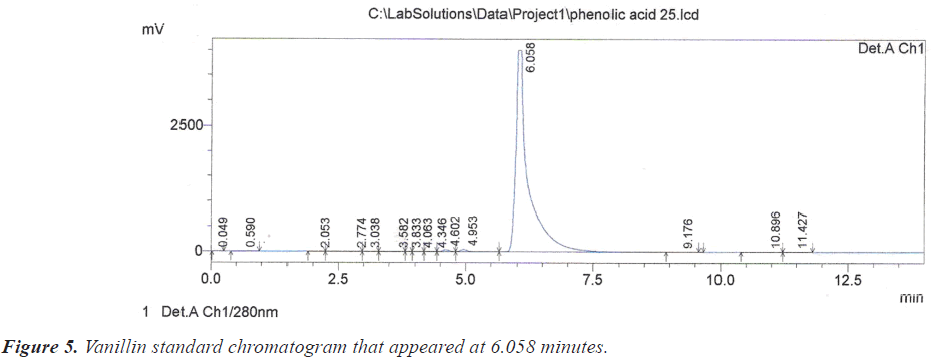
| 6.058 | Vanillin | 3 | 6.067 |
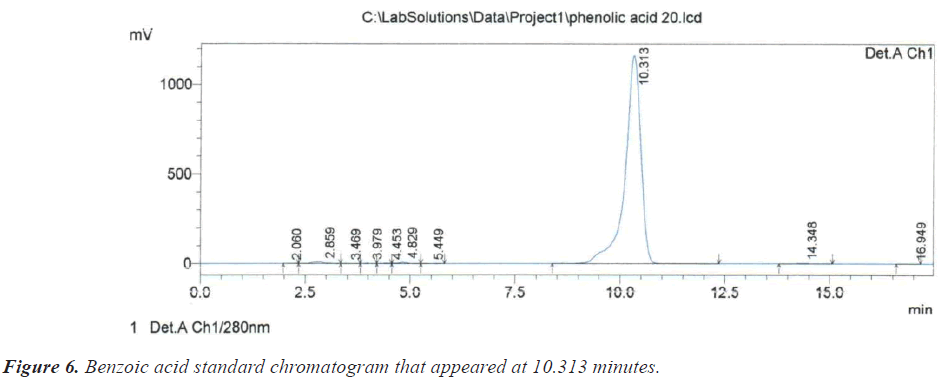
| 10.313 | Benzoic acid | 4 | 10.209 |
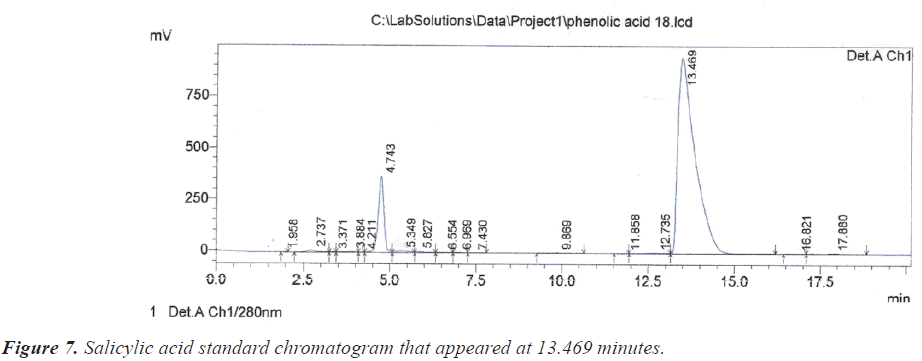
| 13.469 | Salicylic acid | 5 | 13.492 |
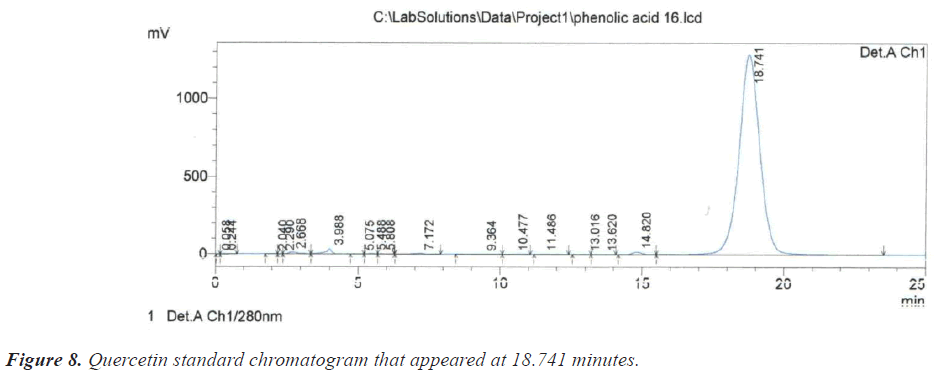
| 18.741 | Quercetin | 6 | 17.99 |
Table 1. The retention time of the compounds under study in HPLC using solvent 50% (A): methanol solution and 50% (B): acetic acid in water (1:25).
Quantitatively, the concentrations of the active compounds under study ranged between (0.06080 and 0.00008) mg/g of plant tissue (Table 2), Ascorbic acid was the highest quantitatively, then Vanillin, Benzoic acid, Catechol, Salicylic acid, While Quercetin was at the end of the series in quantitative terms.
| No | Compounds | Retention time | Area | Height | Area % | Height % | Concentration : (DW of tissue) mg g-1 |
|---|---|---|---|---|---|---|---|
| 1 | Ascorbic acid | 2.725 | 53028836 | 4000246 | 26.232 | 40.446 | 0.06080 |
| 2 | Catechol | 4.895 | 7004824 | 257511 | 3.465 | 2.604 | 0.00045 |
| 3 | Vanillin | 6.067 | 8446574 | 186981 | 4.178 | 1.891 | 0.00065 |
| 4 | Benzoic acid | 10.209 | 3359224 | 69873 | 1.662 | 0.706 | 0.00058 |
| 5 | Salicylic acid | 13.492 | 1794145 | 31307 | 0.888 | 0.317 | 0.00029 |
| 6 | Quercetin | 17.990 | 1091915 | 13188 | 0.540 | 0.133 | 0.00008 |
Table 2. Percentages of compounds which separated during the study.
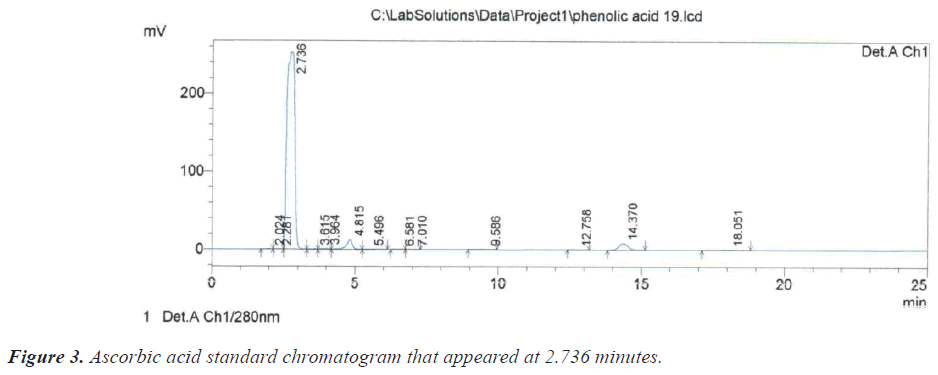
Based on the chromatograms of the standard compounds, the Retention time (Rt) of these compounds adopted as evidence of their presence in the extracts of the sample. On this basis, the peak that appeared at the time 2.725 minutes (Table 1) and (Figure 2) belong to the compound Ascorbic acid because these value are identical to the retention time of 2.736 minutes for the Ascorbic acid standard (Figure 3).
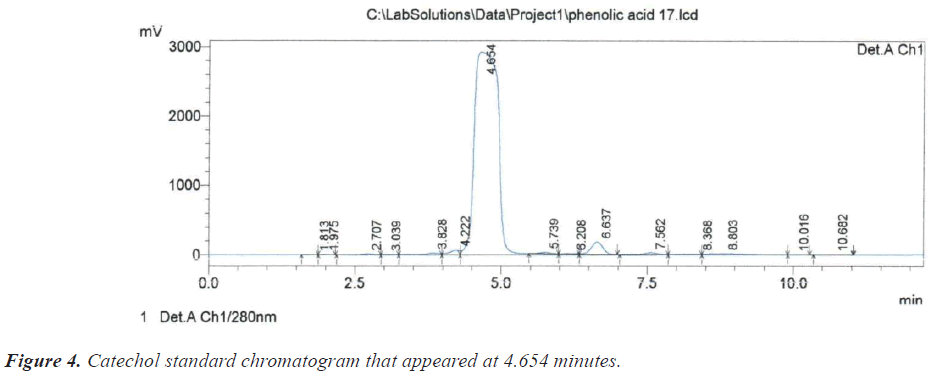
Regarding catechol, Reinsch was able to isolate catechol for the first time in 1839 by distilling it from the solid tannic concentrated juice of Mimosa catechu [19]. During the current study, the retention time of 4.654 may be evidence of absence of catechol in the crude of Sisymbrium irio, but it should be noted that there is a peak at 4.859 minutes, which means that there is a compound that is structurally similar to it, (Figure 4) and (Table 1).
Along with some other chemical compounds Catechol, is used in the treatment of some cancerous diseases that affect the kidneys and liver. Studies have shown that most of the active compounds are found in the form of linked phenols, and they are characterized by having a great diversity in biological activities, the most important of which is the role they play as anti-cancer compounds [20], but they vary in their abundance depending on the type of plant, for example, the presence of simple phenols, such as phenol and thymol, are relatively little in plants, knowing that hydroquinone is the most abundant compared to other simple phenols such as catechol, orcinol, fluorococinol and pyrocalol, so its presence is less [8].
As for the peak that appeared at the retention time of 6.067 minutes on the chromatogram of crud, it is due to vanillin according to the retention time of 6.058 minutes of its vanillin standard (Figure 5).
The importance of the benzoic acid study comes through its important role in preserving food from damage caused by some microorganisms, as it has antimicrobial activity at pH 2.5-4.0. This results in inhibition of the proliferation of bacteria and yeasts, so it is often used in preserving food and soft drinks [21].
As for the peak that appeared at the retention time of 10.209 minutes, it is due to the compound Benzoic acid according to the retention time of 10.313 minutes that appeared on the chromatogram of its standard compound (Figure 6). In the same context, it was noted that there is a large correspondence in the retention time of Salicylic acid (Figure 7) and (Table 1).During the current study, Quercetin, its characterized by the longest retention time (18.741 minutes) compared to other standard compounds (Figure 8), which used evidence for the presence of Quercetin in the studied extracts (Table 1).
Like the rest of the flavonoids to which it belongs, Quercetin has many biological properties, including antioxidant effects, through its ability to scavenge free radicals, which are characterized by having harmful effects on cell membranes, DNA, and often cause cell death. On it Quercetin helps protect the body from cancer and heart disease, as it enhances the stability of cells that produce histamine, which makes it an anti-inflammatory and histamine compound [22].
During the current study, Quercetin, its characterized by the longest retention time (18.741 minutes) compared to other standard compounds (Figure 8), which used evidence for the presence of Quercetin in the studied extracts (Table 1).
It is worth noting that the various phenolic compounds perform many vital functions. For example, binary phenols stimulate growth and so most of them are concentrated in leaves, flowers, fruits and roots, and are resistant to bacteria and fungi attacking plants and thus protecting them against infection with vital diseases, as well as some of them are responsible for the different colors flower petals, thus it attracting insects to flowering plants, which increases the chances of pollination and fertilization, and it also contributes to increasing the rigidity of the mechanical tissues of plants as a result of the formation of lignin from phenyl propanoids units during the polymerization process [23].
Second: The environment study
Table 3 shows that the pH value of the soil under study (8.01) indicates the tendency of the study area to be alkaline, and that this may have an effect on the amount of ready phosphorous in the soil, In this regard, Tisdale and Nelson [24] mentioned that high values of the pH are responsible for the precipitation of phosphorus in the form of calcium phosphate, on the other hand, the soil pH affects plants through its effect on the solubility of some ions such as iron, aluminum and magnesium, whose toxic effects appear when Its presence in high concentrations, as most of the elements are ready at low values, while their damage decreases at pH values close to the base [25].
| Physical Properties | ||
|---|---|---|
| Factors | Concentration | Unit |
| E.C | 1.06 | ds/m |
| Soils particles size | (%) [27] | |
| Clay | 38.31 | |
| Silt | 44.39 | |
| Sand | 17.30 | |
| Chemical properties | ||
| Factors | Concentration | Unit |
| pH | 8.01 | ---- |
| Organic matter | 24.33 | (ppm) |
| Na+1 | 78.0 | (ppm) [28] |
| K+1 | 15 | |
| Ca+2 | 235 | |
| Mg+2 | 47.97 | |
| Cl-1 | 68 | |
| HCO3 -1 | 69 | |
| So4-2 | 187.43 | |
| Ready phosphorous | 1.08 | |
| Ready Nitrogen | 42.12 | |
| Concentrations of trace elements | ||
| Factors | Concentration | Unit |
| Fe | 76.23 | (ppm) [29] |
| Zn | 6.05 | |
| Cu | 0.97 | |
| Pb | 7.46 | |
| Cd | 1.34 | |
Table 3. Chemo physical properties of soil at Al-Qawsiat region.
Electrical conductivity results, which amounted to 1.06 ds.m-1, indicate that the soil under study falls within the natural soil class in which the conductivity values are less than 4 ds.m-1 according to Ryne et al., [26] (Table 1).
During the current study, it was noted that the amount of organic matter, which amounted to 24.33 ppm, is sufficient for the growth of plants, as the soil in desert and semidesert areas contains less than 10 parts per million of organic matter [30] and here it must be noted that Organic matter, even if it is found in soil in relatively small amounts, is still important in soil structure, moisture and biological activities.
During the current study, Concentrations of the positive ions (Na1+, K1+, Ca2+ and Mg2+) were (78,15,235 and 47,97) ppm respectively, while the negative ions (Cl1-, HCO3 1- and SO4 2-) were (68,69 and 187.43) ppm respectively, while the major nutrients (ready phosphorous and ready nitrogen) amounted to (1.08 and 42.12) ppm respectively, and these results indicate that the soil under study is very suitable for plant growth, but it must be said that Most of the positive ions under study were within the low critical limits related to the major elements in the soil mentioned by Almusli et al., [31].
The elements that have a density above the range (5-6) g/ cm3 are known as heavy elements, as their density is five times greater than the density of water, and some of them are characterized by toxicity even at low concentrations such as lead, cadmium and arsenic [32]. It is a source of great concern for environmental workers, as it is linked to human health [33].
Increasing the levels of heavy metals in the plant's nutrient medium may stimulate or inhibit the absorption and distribution of minerals, as well as its negative impact on a number of biological processes such as photosynthesis, which in turn reduces plant productivity [34]. And due to their presence in low concentrations in soil and plants, they are called microelements, and when the soil content of these elements is higher than the permissible limit, the expression pollution with heavy metals is used [35].
With regard to the heavy metals dealt with in the current study (iron, zinc, copper, lead and cadmium), their concentrations were (76.23,6.5,0.97,7.46 and 1.34) ppm respectively, and through these results, the study area did not reach the state of contamination with the elements under study, as it was All of them are less than the values that were determined for the standard soils according to what was mentioned by Aziz et al., [36].
It is worth noting that the studied factors have many environmental impacts that can be summarized in the following paragraphs:
The concentration of lead (7.46) ppm was much lower than (50) ppm determined by the World Health Organization during its publication in 2003, and it is outside the range (0.7-2.93) mentioned by Salim and Fadhel [33] in their study of soils in the industrial areas of Mosul city, and this may be attributed to the proximity of the study area to the Mosul-Dohuk road.
It is worth noting that the percentage of lead Pb ranges between (20-400) µg/liter in natural waters, while in the earth crust, the rate of its presence is 16 mg/kg of soil, Lead enters the automobile fuel industry and then is deposited in soils near car roads and often finds its way to agricultural crops, leaves and fruits [25].
While the cadmium concentration was (1,34) an average case of the range (1-3) ppm, which represents the permissible limit by the World Health Organization, which also indicated that its concentration should not reach 120 mg in a person weighing 70 kg. Al-Sayegh et al., [37] as its presence in relatively high concentrations may cause significant damage to the kidneys and nerves, and may even cause infertility [38], and high concentrations of it may prevent the completion of the hemoglobin biosynthesis process [39].
The increase in the concentration of copper in the environment, indirectly affects the health of the individual, as it may enter the human body through eating plants and meat containing a high percentage of this element. Studies indicate that its concentration can range between 200 and 400 ppm in meat such as liver. Adriano [40] as mentioned earlier that the concentration of copper (0.97) ppm that was obtained in the current study is below the level (20) ppm that was determined for standard soil [41,42].
Soils differ in their content of iron, which constitutes 0.5%- 5% of the weight of the earth’s crust. In the current study, the iron concentration reached 76.23 ppm, which is much lower than the average 350.35 ppm which was observed by Fadhil et al., [39]. It is worth noting that iron has many vital functions as it is included in the composition of respiratory enzymes and is also necessary for oxidation and reduction reactions that occur in the body during various metabolic processes, and it is also included in the composition of the green pigment (Chlorophyll) responsible for building organic matter in autotrophs.
Conclusion
With regard to zinc, it is present in the soil in low concentrations ranging between (10-30) ppm. It is believed that the low pH increases the availability of zinc through the effect of acids in increasing the solubility of both ZnCO3 and ZnS, As well as the rate of erosion of minerals containing zinc. As for calcareous soils or soils in which calcium phosphate is high, these phosphates combine with zinc to form precipitated zinc phosphates, and thus symptoms of zinc deficiency appear on plants growing in that soil.