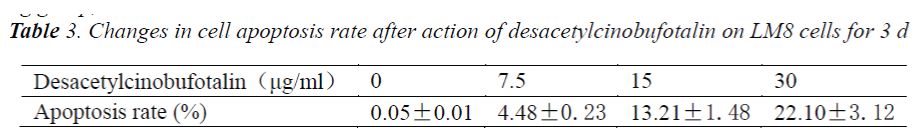ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 1
Study on the chemical constituents of Venenum and the activity of its monomeric compounds against osteosarcoma LM-8 cells.
The Orthopaedic Department, The 82th Hospital of PLA in Huai’an, Jiangsu Province, 223001, China
- *Corresponding Author:
- Songlin Xie
The Orthopaedic Department
The 82th Hospital of PLA in Huai’an
Jiangsu Province, 223001, China
Accepted date: November 30 3014
This paper systematically studied the chemical constituents of Venenum , the effects of Venenum extract and monomeric compounds (Cinobufotalin and desacetylcinobufotalin) on proliferation of osteosarcoma LM-8 cell lines in C3H mice and their relationships with Bcl-2, Bax apoptin expressions were studied, and their mechanisms of apoptosis induction were preliminarily explored. Traditional Chinese medicine Venenum was isolated by silica gel column chromatography, HPLC and recrystallization, and the compound structures were elucidated using modern spectroscopic methods such as 1H-NMR and 13C-NMR, combined with physicochemical properties. WST-8 colorimetric assay was used to detect the effects of 15 μg/ml Venenum extract, Cinobufotalin and desacetylcinobufotalin on growth of LM-8 cell lines; changes in cell morphology were observed; flow cytometry was applied to detect the changes in apoptosis rate; and Bcl-2 and Bax protein expressions were detected. The structural elucidation revealed that the two compounds isolated were cinobufotalin and desacetylcinobufotalin. Desacetylcinobufotalin could significantly inhibit the growth of LM-8 cells, and the effect was dependent on concentration and time. Desacetylcinobufotalin can induce apoptosis of LM-8 cells, and the action was dose dependent, the mechanism is achieved by up-regulation of Bcl-2 expression and down-regulation of Bax expression.
Keywords
Venenum ; Desacetylcinobufotalin; 13C-NMR; 1H-NMR; Osteosarcoma LM-8
Venenum was originally recorded in the "Ben Cao Yan Yi (Augmented Materia Medica)", which is a precious Chinese medicine, it is also known as Chan Su Mei Zhi ("Theory of Drug Properties"). Venenum is the dried secretion of Bufo bufo gargarizans Cantor or B. melanostictus Schneider. Venenum is acrid, warm, toxic, and has detoxification, swelling subsiding, refreshing and resuscitation inducing effects. Recent studies have found that Venenum has a good anti-tumor effect [1-3], which has been widely used in the clinical treatment of various cancers and tuberculosis.
Osteosarcoma is one of the most common primary bone malignancies, which occurs mostly in adolescence, with an extremely high mortality, causing great harm to the health of young people [4]. The majority of drugs used currently for the treatment of osteosarcoma have strong toxic and side effects, the finding of novel safe and effective drugs can improve the treatment efficacy of osteosarcoma [5]. In this study, cinobufotalin and desacetylcinobufotalin were acted on osteosarcoma LM8 cell lines, CCK-8 assay and flow cytometry were used to study their effects on apoptosis of osteosarcoma cells, and immuno histochemistry techniques were applied to preliminarily explore their apoptosis inducing mechanisms.
Materials
Drugs and reagents
Venenum powder was purchased from Chengdu Biotechnology Co., Ltd. (batch number: 20130812), which was identified by Professor Chen Ming of Chengdu University of TCM as Bufo bufo gargarizans Cantor. Chloroform, methanol, petroleum ether (60-90°C), acetone, ethyl acetate were chemically pure reagents, which were purchased from Chongqing Chemical Reagent Company. C3H mouse osteosarcoma cell line LM-8, RPMI1640 medium, fetal calf serum, Bcl-2 and Bax antibodies, DAB staining kit and SP kit were all purchased from Wuhan Biotechnology Co., Ltd.
Instruments
Yanaco MP-S3 micro melting point apparatus (uncorrected), Japan; BRUKER-AV400 NMR spectrometer (TMS internal standard); analytical balance (Nanjing Kanglong Electronic Weighing Instrument Co., Ltd.); thermostatic incubator (Forma Scientific); FACS420 flow cytometer; Olympus inverted microscope.
Materials and Methods
Extraction and isolation of chemical constituents of Venenum
1 kg of Venenum powder was taken, and extracted under reflux with 95% ethanol for 1.5 h three times, after ethanol was recovered until no ethanol smell was detectable, the remaining was dissolved in an appropriated amount of water, and extracted three times with an equivalent volume of CHCl3 to give 70 g of chloroform layer, as well as 60 g of aqueous layer. The chloroform extract was subjected to isolation methods of silica gel column chromatography, HPLC and recrystallization purification to yield cinobufotalin (1) and desacetylcinobufotalin (2).
Structural elucidation of cinobufotalin and desacetylcinobufotalin
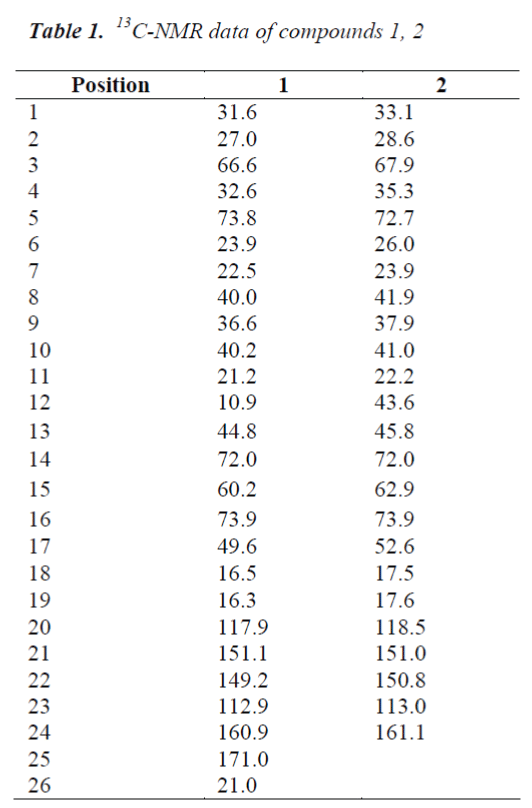
Compound 1, white needle crystals (methanol), in 1HNMR( 400MHz, CDCl3), δ: 7.78(1H,m), 7.20(1H,brs) and 6.12(1H,d,J=8.0Hz) were three unsaturated carbon hydrogen signals, indicating that the compound may be proton signal on unsaturated six-membered lactone ring of cardenolide B; chemical shifts at δ:5.43(1H,d,J=12.0Hz), 4,31(1H,s) and 33.59(2H,s) were proton signals on three oxygen-bearing carbons; and δ: 0.81(3H,s) and 0.99(3H,s) were C-18, 19 angular methyl group proton signals on the nucleus, respectively. δ:1.90(3H,s) was methyl proton signal on the ester carbonyl group, combined with C NMR data, the presence of an acetyl group was indicated in the structure. 13C-NMR(100MHz, CDCl3) gave a total of 26 carbon signals, including a group of acetyl carbon signals δ:171.0, 21.0; δ: 160.9 lactone carbonyl carbon signal and four unsaturated carbon signals δ:151.1, 149.2, 117.9 and 112.9 which were assigned to the C-21, 22, 20 and 23 positions of cardenolide B, respectively; among the five oxygenated carbon signals, δ:72.0 and 60.2 were assigned to C-14, 15 positions, suggesting that C-14, 15 positions were epoxy structures, the other three oxygenated carbon signals δ:73.8, 73.9 and 66.6 were assigned to C-16, 5 and 3, respectively.
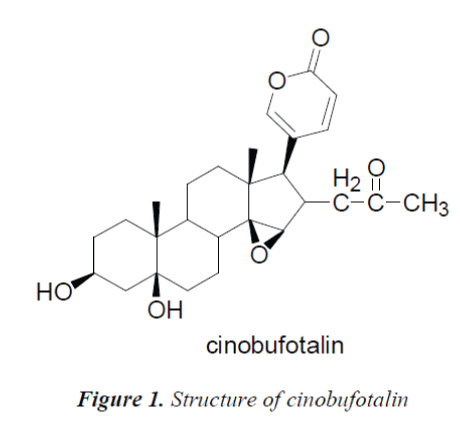
The C, H spectral data of the compound were consistent with those of cinobufotalin reported in the literature [6], so the compound was identified as cinobufotalin, its structure was shown in Fig. 1, and 13C-NMR data in Table 1.
Cell culturing
RPMI1640 containing 10% fetal calf serum was used as the culture medium, and cells were placed in a 37°C, 5% CO2 incubator for incubation. After about 3 days when the cells covered more than 80% of the bottom of culture flask, they were digested with 0.25% trypsin and 0.02% EDTA. After being digested into single cells, the cells were seeded in culture flasks at 5×105 cells/flask.
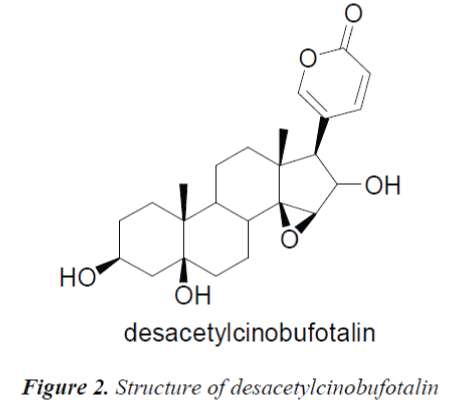
Compound 2, white needle crystals (methanol), in 1HNMR( 400MHz, pyridine-d5), δ:8.42 (1H, d, J=9.6Hz), 7.28(IH,br.s) and 6.10(1H, d, J=l0.0Hz) were three unsaturated carbon hydrogen signals, suggesting that the compound was proton signal on unsaturated sixmembered lactone ring of cardenolide B; chemical shifts at δ:4.87 (1H, d, J=9.2Hz), 4.22 (1H, s) and 3.79 (1H, s) were proton signals on three oxygen-bearing carbons; and δ:0.88 (3H, s) and 1.14(3H, s) were C-18, 19 angular methyl group proton signals on the steroid nucleus. 13CNMR( 100MHz, Pyridine-d5) showed a total of 24 carbon signals, lactone carbonyl carbon signal at δ: 161.1 and four unsaturated carbon signals δ:151.0, 150.8, 118.5 and 113.0 were assigned to the C-21, 22, 20 and 23 positions of cardenolide B, respectively; among the five oxygenated carbon signals, δ:72.0 and 62.9 were assigned to C-14, 15 positions, suggesting that C-14, 15 positions were epoxy, the other three oxygenated carbon signals δ:73.9, 72.7 and 67.9 were assigned to C-16, 5 and 3, respectively. The C, H spectral data of the compound were consistent with those of desacetylcinobufotalin reported in the literature [6], so the compound was identified as desacetylcinobufotalin, its structure was shown in Fig. 2, and 13C-NMR data in Table 1.
Cells in logarithmic growth phase were digested With 0.25% trypsin and 0.02% EDTA, and cell concentration was adjusted to 5×104/ml, then the cells were added to the culture plate, added with an appropriate amount of the above culture medium, and cultured in a 37°C, 5% CO2 incubator until completely covering the plate.
Grouping
C3H mouse LM-8 osteosarcoma cells in the logarithmic growth phase were digested into suspension with 0.25% trypsin and 0.02% EDTA. The suspension was passaged in the culture flask, when the cells covered about 70% of the flask, the original medium was discarded and replaced by a new medium containing Venenum extract, cinobufotalin and desacetylcinobufotalin, drug concentration was 15 μg/ml in all of the groups, then the culturing was continued for an additional 24 h.
Measurement of the inhibitory effects of Venenum extract, cinobufotalin and desacetylcinobufotalin on LM-8 osteosarcoma cell proliferation by WST-8 assay
The logarithmic growth phase C3H mouse LM-8 osteosarcoma cells were prepared into an 80,000 cells/ml suspension. 100 μl of cell suspension was added to each well of culture plates, and placed in a 37°C, 5%CO2 incubator for preincubation. The culture medium in each well of the culture plates was replaced by 10 μl of new medium containing Venenum extract, cinobufotalin and desacetylcinobufotalin. Drug concentration in each group was 15 μg/ml. The plates were placed in a 37°C, 5%CO2 incubator and cultured. At 24, 48 and 72 h, 10 μl of CCK- 8 solution was added to each well. After an additional 6 h of culturing, optical density was measured at 450 nm using automatic microplate reader. Cell growth inhibition rate in each group was calculated according to the optical density.
Cell proliferation inhibition rate (%) = [(Ac-As) / (Ac- Ab)] × 100%
As: experimental wells (drug-containing medium)
Ac: control wells (drug-free medium)
Ab: blank wells (cell- and drug-free medium)
The concentration of drug at which cell growth was inhibited by 50% (IC50) was calculated using IC50 calculator software, and the analysis of variance on the experimental data were performed using SPSS 11.5 statistical package.
Measurement of apoptosis by flow cytometry
Adherent cells were washed with 0.01 mol/L PBS, and digested with 0.25% trypsin and 0.02% EDTA. The digested cells were added to the culture medium, slightly mixed, and centrifuged for 5 min, then the supernatant was discarded, and cells were collected and counted. 80,000 resuspended cells were taken, centrifuged for 5 min, then the supernatant was discarded, and 195 μL of Annexin V-FITC binding buffer was added to gently resuspend cells. 5 μL of Annexin V-FITC was added. The cells were incubated in the dark at room temperature for 10 min, centrifuged for 5 min, then the supernatant was discarded, and 190 μL of Annexin V-FITC binding buffer was added to gently resuspend cells. The cells were then added with 10 μL of propidium iodide (PI) staining solution, mixed gently, and placed in dark place in an ice bath. Flow cytometry was performed forthwith, Annexin VFITC was green fluorescence, and PI red fluorescence. The results demonstrated that after Annexin V-FITC and PI staining, the normal viable cells were not stained with Annexin V-FITC or PI; early apoptotic cells were only stained with Annexin V-FITC, which displayed negative PI staining; late apoptotic cells and necrotic cells could be simultaneously stained with Annexin V-FITC and PI. Analysis of variance on the experimental data was performed using SPSS 11.5 statistical package.
Cell immunohistochemistry experiment
Culture plates which were treated by drugs for 72 h were removed, rinsed, and culture medium was decanted, cell slides were taken out, and washed with 4°C PBS. 4% paraformaldehyde fixative was added, and the slides were fixed for 15-20 min at 4°C. The specimens were washed with 0.01 mol/L PBS. 0.3% methanol-hydrogen peroxide solution was added and the slides were incubated at room temperature for 20 min. The specimens were washed with 0.01 mol/L PBS, then blocked with goat serum blocking solution and 0.3% TritonX100 for 30 min. Serum was decanted, and 1:100 Bcl-2 and BaX were added separately, and the slides were kept overnight at 4°C in a humid chamber. The slides were then rinsed with 0.01 mol/L PBS, added dropwise with 1:400 diluted second antibody, and incubated at 37°C in a humid chamber for 30 min. The slides were rinsed with 0.01 mol/L PBS, added dropwise with ABC complex, and incubated at 37°C in a humid chamber for 30 min. The slides were rinsed with 0.01 mol/L PBS, and visualized at 37°C with DAB chromogenic kit, reaction time was controlled microscopically at around 3~5 min till positive cells were brown. Slides were then dehydrated with ethanol series, cleared with xylene for 2 min, and mounted in neutral resin, followed by observation under microscope and recording. 10 relatively well stained fields were randomly selected in each experimental group, respectively, and photographed under microscope, gray values of the staining results were measured using Image-pro Plus. Analysis of variance on the experimental data was performed using SPSS11.5 statistical package.
Experimental Results
Effects of Venenum extract, cinobufotalin and desacetylcinobufotalin on LM-8 cell morphology
15 μg/ml Venenum extract, cinobufotalin and desacetylcinobufotalin were acted on LM-8 cells for 72 h, respectively, the results showed changes in cell morphology, such as decreased adherent cell density, increased floating cells, irregular cell shape, and partial cell fragmentation.
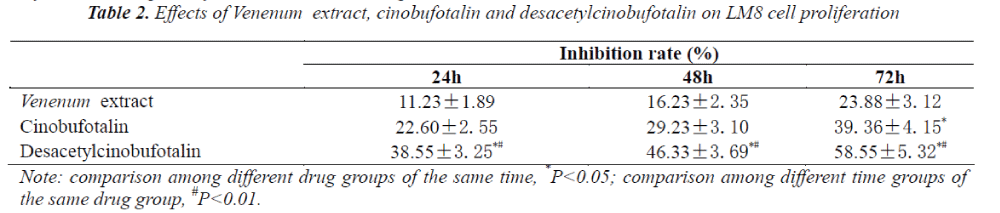
Inhibitory effects of Venenum extract, cinobufotalin and desacetylcinobufotalin on LM-8 cell proliferation
The optical density of each well of cells was measured under different action time of different drugs based on WST-8 assay, and respective cell growth inhibition rates were calculated, the results are shown in Tab. 2. It can be seen from the results that the effect of desacetylcinobufotalin on LM-8 cells was apparent, compared with other groups, its LM-8 cell proliferation inhibitory effect was time dependent, while the effects of Venenum extract and cinobufotalin were not obvious.
By factorial design analysis of variance, the cell growth in inhibition rate of desacetylcinobufotalin was significantly different at different times (P<0.05). After calculation, IC50 values of 1st, 2nd, 3rd days were 23. 50μg/ml, 16. 00μg/ml and 12 .09 μg/ml, respectively.
Effect of desacetylcinobufotalin on apoptosis rate of LM-8 cells
In the early apoptotic phase, phosphatidylserine (PS) migrated from the inner cell membrane to the outer cell membrane. Annexin V-FITC showed high affinity for PS, during apoptosis before DNA fragmentation, PS was exposed, and apoptotic cells showed resistance to PI staining, while necrotic cells didn't. When the cell membrane damage occurred, the cell's DNA could be PI stained to generate red fluorescence, while the cells with intact cell membrane did not produce red fluorescence. Therefore, in the early apoptotic phase, cells will not be stained by PI so red fluorescent signal will not appear. After treating LM-8 cells with different concentrations (0 μg/ml, 7.5 μg/ml, 15 μg/ml and 30 μg/ml) of desacetylcinobufotalin for 72 h, the apoptosis rate changed markedly. With the increase of desacetylcinobufotalin concentration, apoptotic cells also increased. The data are shown in Tab. 3. The analysis of variance showed significant difference (P<0.05) in the apoptosis rate among different desacetylcinobufotalin concentration treatment groups, suggesting that the apoptosis inducing effect of desacetylcinobufotalin was positively correlated with the drug concentration within the experimental range.
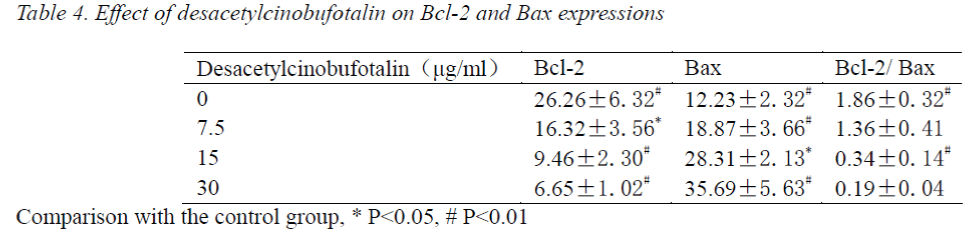
Effect of desacetylcinobufotalin on Bcl-2, Bax protein expressions
After treating with different concentrations of desacetylcinobufotalin for 72 h, the LM-8 cells were stained by SP, and photographed under microscope, the staining results were analyzed using Image-pro Plus, 20 positive cells were selected from each part of each section for gray value measurement, statistical data are shown in Tab. 4. The factorial design analysis of variance showed that Bcl-2 expression was relatively strong, but gradually decreased with the increase of desacetylcinobufotalin concentration (P<0.05); while Bax expression was precisely the opposite, which increased gradually with increasing desacetylcinobufotalin concentration (P<0.05), the ratio between the two also decreased gradually (P<0.05), suggesting that desacetylcinobufotalin could alter the protein expression of apoptotic genes of LM-8 cell
Discussion
Venenum has complex chemical composition, in the present experiment, bufogenin monomer compounds were isolated through systematic study of chemical constituents, providing the material basis for further study of pharmacological activity. However, up to now, high polar chemical constituents of Venenum have been scarcely reported, for more systematic development of Venenum , in-depth study of high polar chemical constituents and their pharmacological activity is necessary.
In this experiment, the osteosarcoma cell killing effects of Venenum extract, cinobufotalin and desacetylcinobufotalin were studied, the study found that after treatment by desacetylcinobufotalin, in vitro cultured LM-8 osteosarcoma cells presented irregular shape, large amount of cell debris was produced, adherent density of cells decreased, floating cells appeared, and partial cell death was found. The effect of desacetylcinobufotalin on LM-8 cell proliferation was observed by WST-8 assay, which found that desacetylcinobufotalin had an inhibitory effect on in vitro cultured osteosarcoma cells in a time-dependent manner. Flow cytometry analysis showed that after treatment of LM-8 cells by desacetylcinobufotalin, apoptosis proportion gradually increased with increasing drug concentration, suggesting that desacetylcinobufotalin can induce LM-8 cell apoptosis, and the strength of apoptosis inducing effect was positively correlated with drug concentration.
Currently, there are two classical pathways of apoptosis which are widely accepted [7-10], they are death receptor pathway and mitochondrial pathway, Bcl-2 protein family plays a crucial role in the mitochondrial pathway, its antiapoptotic protein Bcl-2 and pro-apoptoticprotein Bax are both localized in the mitochondrial membrane, during the process of apoptosis induction, Bax content increases, which transfers from the cytoplasm to the mitochondrial outer membrane, changing the permeability of the mitochondrial membrane, leading to mitochondrial channel (PTP) opening, mitochondrial transmembrane potential decreases, allowing CytoC to release into the cytoplasm and activate downstream caspase enzymes, initiating caspase cascade reaction, and ultimately cause apoptosis [11]. While the anti-apoptotic protein Bcl-2 can prevent Bax shift to the outer mitochondrial membrane, or directly act on the mitochondrial channels and thereby block CytoC release. According to the relevant literature [12], the cells' sensitivity to death signals depends on intracellular competitive dimerization process of Bcl-2/Bax, Bax dimer formation can induce apoptosis, while increased expression of Bcl-2 protein can separate Bax dimers, and form Bax-Bcl-2 dimers which are more stable than the Bax-Bax, antagonizing the Bax-Bax-induced apoptosis, so that cells do not undergo apoptosis. A study has further confirmed this conclusion [13], in which primary osteosarcoma and non-malignant bone tissue specimens were immunohistochemically stained to study the expression of apoptosis-related genes, the results found that p53, Bax and Bcl-2 were relatively highly expressed in tissues of osteosarcoma patients, while not expressed in benign control group. Statistics have shown that patients with lower Bcl-2/Bax have low 4-year survival rate, and short disease-free survival. All of the patients whose expression of apoptosis-related genes was Bcl-2(- )/Bax(+)/p53(+) had a recurrence within four years. It can be speculated that Bcl-2/Bax ratio is an important factor in determining the occurrence of apoptosis, during the onset and development of osteosarcoma, cells not only undergo abnormal proliferation, more importantly, their ability and propensity to apoptosis also decrease, presenting elevated level of Bcl-2 expression, and decreased Bax expression. Up-regulation of Bcl-2 or down-regulation of Bax can inhibit osteosarcoma cell apoptosis induced by a variety of factors. Conversely, down-regulation of Bcl-2 or up-regulation of Bax promotes apoptosis in osteosarcoma cells.
In this paper, our investigation of Bcl-2 and Bax proteins found that Bcl-2 protein expression, which promotes osteosarcoma cell survival, was highest and strongest in the control group, which gradually decreased with increasing desacetylcinobufotalin concentration, while the proapoptotic Bax protein expression was the lowest and weakest, which was elevated and strengthened gradually with increasing desacetylcinobufotalin concentration. Bcl- 2/Bax protein ratio indicates the state of osteosarcoma cell survival, which decreases gradually with the increase of desacetylcinobufotalin concentration. All in all, the changes in Bcl and Bax proteins after treatment of osteosarcoma cells by desacetylcinobufotalin all indicate that desacetylcinobufotalin can induce apoptosis of osteosarcoma cells.
In conclusion, we demonstrated the apoptosis inducing effect of desacetylcinobufotalin on osteosarcoma cells from the morphological and gene protein level perspectives, moreover, the apoptosis inducing effect showed dose-dependency, its mechanisms involve up-regulation of Bcl-2 expression and down-regulation of Bax expression, desacetylcinobufotalin will be an ideal antiosteosarcoma drug with development potential.
References
- Numazawa S, Inoue N, Nakura H, Sugiyama T, Fujino E, Shinoki M. A cardiotonic steroid bufalin-induced differentiation of THP-1 cells. Involvement of Na+, K+- ATPase inhibition in the early ehanges in Protooncogene expression. Biochem Pharmacol 1996; 52: 321-329.
- Watabe M, Ito K, Masuda Y, Nakajo S, Nakaya K. Activation of AP-1 is required for bufalin-inducede apoptosis in human leukemia U937 cells. Oncogene 1998; 16: 779-787.
- Xie L, Chang Q. The Pharmacological action and Praeparatum development of Chan su. Vet Pharm Feed Addit 2002; 7: 26-27.
- Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar- Graney K, Shmookler B, Malawer MM. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician 2002; 65: 1123-1132.
- Bacci G, Lari S. Adjuvant and neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov 2001; 86: 253- 268.
- Wang JD, Narui. Hematological studies on naturally occurring substances. VI effeets of an animal crude drug “chan su” on blood coagulation, fibrinolysis system and cytoxicity. Chem Pham Bull 1991; 39: 2135- 2137.
- Wong WW, MacDonald S, Langler RF, Penn LZ. Novel synthetic organosulfur compounds induce apoptosis of human leukemic cells. Anticancer Res 2000; 20: 1367- 1374.
- Sigounas G, Hooker JL, Li W, Anagnostou A, Steiner M. S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutr Cancer 1997; 28: 153-159.
- Sengupta, Ghosh S, Bhattacharjee S, Das S. Indian food ingredients and cancer prevention - an experimental evaluation of anticarcinogenic effects of garlic in rat colon. Asian Pac J Cancer Prev 2004; 5: 126-132.
- Sun L, Wang X, Pan YJ. Effects of allicin on telomerase activity and apoptosis in gastric cancer SGC-7901 cells. Medical Journal of Chinese People's Liberation Army 2003; 28: 445-446.
- Green DR, Reed JC. Mitochondrion and apoptosis. Science 1998; 281: 1309-1312.
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995; 80: 285-291.
- Kaseta MK, Khaldi L, Gomatos IP, Tzagarakis GP, Alevizos L, Leandros E, Papagelopoulos PJ, Soucacos PN. Prognostic value of bax, bcl-2, and p53 staining in primary osteosarcoma. J Surg Oncol 2008; 97: 259- 266.