ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-I
Study on the effect of soil beneficial microorganisms on the growth of kiwifruit
1Research Institute for Special Plants, Chongqing University of Arts and Sciences, Chongqing, PR China
2College of Forestry and Life Science, Chongqing University of Arts and Sciences, Chongqing, PR China
- *Corresponding Author:
- Huihe Li
Research Institute for Special Plants
Chongqing University of Arts and Sciences
PR China
Accepted date: January 22, 2017
In our country, the consumption demand of the kiwifruit has increased year by year. Every year, we need to import a large amount of kiwifruit from abroad to meet the domestic consumption demand. In order to improve the growth of kiwifruit, promote its good growth and development, and improve the yield, study on the effect of beneficial microorganisms on the growth of kiwifruit has a very important significance. By setting up the control analysis experiment, the experimental group and the control group were compared and analyzed, in order to explore the specific effects of beneficial microorganisms on the growth of kiwifruit. The experimental group was added with nitrogen fixing bacteria, phosphate dissolving bacteria and potassium bacteria. In addition, the growth environment of the control group and the experimental group was the same, and the growth of the plant of kiwifruit was determined. The culture was developed over a period of 90 days. Through the comparative analysis, it can be seen that the growth and development of the experimental group with beneficial microorganisms in the soil was better than that of the control group, and the disease resistance was better, which could absorb the elements such as Nitrogen (N), Phosphorous (P), Potassium (K) and so on. It can be concluded that the beneficial microorganisms in optimized soil can contribute to the growth and development of kiwifruit, and it can be used in the production practice.
Keywords
Kiwifruit, Beneficial microorganism, Microbial fertilizer
Introduction
During the year of 2010-2015, with China's rapid development of the kiwi industry, the average annual growth rate of planting area reached 10%. In 2015, the annual planting area was more than 2 million 250 thousand acres, and the total yield of kiwi was nearly 1 million 200 thousand tons. However, China's consumption of kiwifruit is also increasing year by year. The total output of the domestic kiwifruit is difficult to meet domestic consumption, and it needs need to be imported from abroad yearly [1]. Therefore, only to increase the planting area of domestic kiwifruit is difficult to fully meet the domestic demand. Improving the growth and development of kiwifruit, and reducing the incidence of the disease are needed to improve the yield of kiwifruit. Although the traditional fertilization means can effectively promote the growth and development of kiwifruit, there are certain modifying effect on soil, which is not conducive to soil microbial environment and long-term sustainable development, with only obtaining short-term benefits. The way to reduce the prevalence of kiwifruit by pesticide spraying can not only cause heavy metal pollution in the environment, but also can be harmful to the human body. Therefore, more scientific and reasonable, healthy and sustainable biological means are needed to promote the growth and development of the kiwi, and reduce the possible incidence. In recent years, microbial fertilizer has become more and more widely used in agricultural applications. And a lot of practice has proved that it has a very positive effect on the plant growth and yield increase [2]. Microbial fertilizer is used to optimize the microbial in the soil, and to further improve the effect of the beneficial microorganism on the plant. Microbial life activities are used to continuously improve the survival environment of plant rhizosphere and the absorption of nutrient elements.
However, there is still a lack of sufficient experiments to study the effect of microorganisms on the cultivation of kiwifruit. Kiwifruit is rich in N, P, K, Ferrous (Fe) and other mineral elements, as well as other various beneficial nutrients to the body, and it needs to absorb a large number of nutrients from the soil [3]. Therefore, more experiments are needed to explore the effects of microorganisms on the growth of kiwifruit, so as to provide theoretical basis for the application of microbial fertilizers in the growth of kiwifruit. The optimization of microorganisms in soil can give full play to the role of various soil microorganisms promote plant growth, and improve the yield and quality of kiwifruit. At the same time, it can reduce the pollution of the environment, in order to make the soil sustainable use [4]. Therefore, through the contrast experiment, artificially added beneficial bacteria are used to improve the soil microbial community in the growth process of kiwifruit. Then the growth situation, disease resistance and the contents of all kinds of elements contained in the plant are analyzed for the comprehensive evaluation, so as to further analyze the effect of beneficial microorganisms on the growth of kiwifruit.
In order to avoid the effects of other nutrients, air, water, humidity, and light on the growth of two groups of kiwifruit, the growth environment of both the experimental group and the control group are all consistent, and the random interval distribution of kiwifruit is made in order to reduce the influence of the environmental difference. Because the growth cycle of kiwifruit is long, the contrast experiment is only carried out for 90 days. Although the whole growth process of the seedlings is observed, statistics of the fruit yield and the analysis of fruit quality are not conducted. In the follow-up study, it is necessary to further extend the experimental time, so as to obtain the full cycle data of the growth and development of kiwifruit, in order to optimize the effects of beneficial microorganisms on the growth of kiwifruit.
Huang et al. [5] studied the impacts of combining N-fixing species with Eucalyptus on Microbial Biomass Carbon (MBC) as well as Phospholipid Fatty Acid (PLFA) composition through usage of a comparative experiment of an eight year old pure Eucalyptus urophylla plantation (PP) as well as an eight year old mixed E. urophylla as well as Acacia mangium plantation (MP) in Guangxi Zhuang Autonomous Region of southern China. Moreover, a trench control experiment was carried out for distinguishing the impacts of roots on MBC as well as community structure. The outputs reveal that the quantity as well as huge presence of PLFAs as well as MBC were greater in MP than in PP. The soil TOC, NH4-N, NO3-N, TN as well as litterfall mass were considerably more in surface soil (0-10 cm) in MP; while the C/N ratios of soil as well as litterfall were considerably lesser by 37.6% as well as 78.5%, correspondingly, in MP than in PP. MBC at the surface soil (0-10 cm) was considerably more by 9.0% in MP than in PP. Principal Component Analysis (PCA) showed that soil microbial community structure was different between the 2 plantations, as well as between the trenched as well as untrenched plots. Redundancy analysis (RDA) further showed that C/N ratios of soil as well as litterfall, TOC, NH4-N, litterfall mass as well as NO3-N were important causes impacting the soil microbial community disregarding the plantation types.
García-Orenes et al. [6] focuses on assessing the impact of various agricultural management practices on soil microbial community structure and assessed as huge presence of phospholipid fatty acids, PLFA. 5 distinct treatments were chosen, on the basis of the most typical practices utilized by farmers in the studied region: residual herbicides, tillage, tillage with oats as well as oats straw mulching; such agricultural practices were assessed against discarded land post farming as well as a nearby long term wild forest coverage. The outcomes prove that a considerable level of differentiation in the microbial community structure, with respect to management practices, that was extremely related to soil organic matter content.
Growth Law of the Kiwifruit and the Effect of Microorganisms on the Growth of Plants
Growth law of kiwifruit
Because of its rich nutrients, kiwifruit is no longer used as a kind of people's daily consumption of fruit, and it has been gradually developed into a new type of health food. China is a big country of kiwifruit production, and it is also an important consumer of kiwi, people are very popular with the kiwi [7]. This also leads to that the domestic production of Chinese kiwifruit is difficult to fully meet the needs of the domestic people. Every year a large number of kiwifruit is needed to be imported from abroad, and the main importing countries include Australia, New Zealand and Italy, etc. [8].
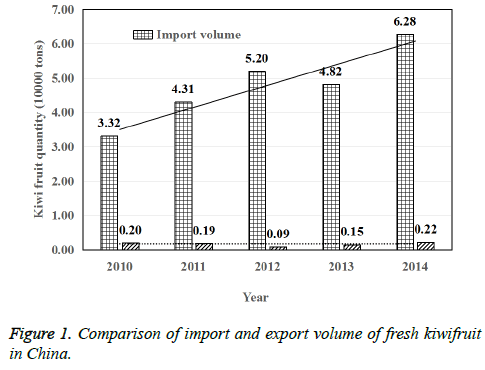
During the period of 2010-2014, the import and export volume of fresh kiwifruit in China was shown in Fig. 1. It can be seen from Figure 1, China's exports of fresh kiwifruit was significantly less than the number of imports. And according to the 5 years of statistics, China's import of kiwifruit was increasing year by year, while the number of exports was no significant change. Taking the year of 2014 for example, China's exports of fresh kiwifruit was 2 thousand and 200 tons, and the import volume was 62 thousand and 800 tons, so the imports were 28.5 times the amount of exports [9]. Therefore, on the basis of the guarantee of the planting area, improving the growth quality of kiwifruit and reducing the disease in the growth process is one of the main tasks for kiwifruit in China. It is observed from fig 1 that in the year 2011, the import of kiwi fruit (in tons) increased by 25.95% than 2010, in 2012 it is increased by 18.72% than 2011, in 2013 it is decreased by 7.58% than 2012 and in year 2014 it is increased by 26.31% than 2013.
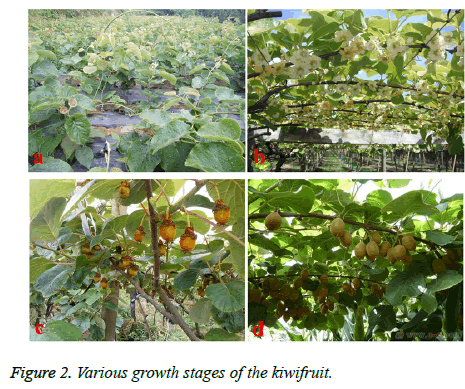
The growth cycle of kiwifruit generally consists of four stages (Figure 2), respectively is the seedling growth period (Figure 2a), flowering stage (Figure 2b), fruit growth period (Figure 2c) and fruit maturity (Figure 2d). Each growth stage is mainly reflected in the main roots, stems and fruits. According to the performance of the three aspects, the growth of the whole plant can be judged [10]. The root of the kiwifruit is fleshy root. The skin is thick and easy to fall off, and the endothelium is pink. Because the kiwi root shaped tube is relatively developed, the roots have a strong migration ability of nutrient and moisture, and there will be big bleeding when the roots are cut off. Compared to shoot, the root growth and development cycle is longer, more complex, and its effect is most obvious. The peak of root growth is about 20°C, and the second growth and development will carry out after the fruit is ripe in September [11]. The kiwifruit belongs to the lianas, so the seedling stems and twigs have creeping growth, with the growth generally in the direction of counter clockwise spiral. In the subtropical region, the growth period is greater than 6 months. At the end of May, in early September, when the temperature is appropriate, there will be a growth peak. And subtropical regions generally have two to three growth peaks. After the flowering period, the kiwifruit will officially enter into the growth period of the fruit. Generally, the kiwifruit has three growth stages: the rapid growth period, slow growth period and the weak growth period. During the 10 weeks after the flower, fruit increases rapidly, and the volume and weight of the fruit can grow to 2/3 of the total amount of maturity. During the slow and weak growth period, with the small changes of the volume and weight, the fruit seeds become light brown and gradually deepened, full, with rapid increase in nutrient concentrations [12].
The kiwifruit is rich in mineral elements such as N, P and K, which not only directly affect the nutritional value of the kiwifruit, but also are closely related to its taste and storage. Among them, N is an important component of cell nucleic acids, hormones, vitamins, and so on. The N element supply also affects the formation of the plant body. This is because the rapid growth of seedling stage of fruits and vegetables needs a large amount of N element, and it will affects the whole tree roots, flowers, fruits and leaves of all tissue growth [13]. P element constitutes a number of important compounds in the plant, and it is very important for photosynthetic phosphorylation and carbon assimilation which are related with the photosynthesis. In addition to the structure of the cell, P has played a very important role in the metabolism of the kiwifruit. And with the lack of supply of P element, it will make the kiwifruit growth slow and even stagnant growth. Like N and P, K is also one of the essential nutrition elements in all stages of the growth and development of fruit and vegetables. K is involved in the composition of organic matter, and it is the activator of many enzymes, to promote fruit development, and contribute to the improvement of yield and quality.
In the planting of kiwi, we need to select the appropriate soil. Only non-sticky and non-alkaline soil are suitable for the growth of kiwifruit, such as red soil, brown soil, yellow soil, mountain meadow, mountain soil and sandy loam soil [14]. The pH value of soil is 5.5-6.5, and the soil is rich in N, P, K, Mg and other elements. And the humus content is high, with better water holding capacity and good ventilation. When the soil shows a neutral and alkalescent, kiwifruit seedling growth will be very slow, and the chlorosis may appear. Because of this, in the weak alkaline soil for the planting of kiwifruit, the modification of soil properties should be done with artificial fertilization, so that the environment is more suitable for its growth [15].
Effects of soil microbes on plant growth
Because the soil is rich in water, air and rich nutrients, it can not only provide suitable growth environment for plant growth, but also can provide a good environment for the life of microorganisms. Due to the different soil pH, humidity, organic matter content, both the types and the number of microbial species are also different in different soils [16].
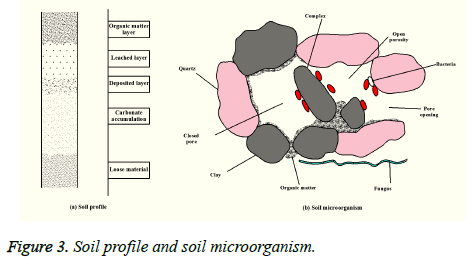
The soil profile from soil surface to the bottom is generally divided into the organic layer, leaching layer, deposition layer, carbonate leaching and accumulation of loose material. In different soil profiles, the microbial species and distribution also have a greater difference. Figure 3 is a typical distribution of microorganism and soil. Plant residues, microbial and clay mineral particles reunite together after many combinations. Microorganisms include bacteria, fungi, actinomycetes, algae, etc. But it is generally content with the decline law that bacteria>actinomycetes>mold>yeast>algae. In different soils, the bacterial content is usually the most, and the dry weight of bacteria is about 1% of the organic matter content in the soil. These microorganisms and plant rhizosphere form a special ecological circulation system, which has great influence on the absorption and transformation of nutrients for plants, thus affecting the growth and development of plants [17].
Microorganisms have a positive effect on plant growth. During the period of their life, it is possible to decompose the insoluble minerals in the soil, which can be transformed into the easily soluble mineral compounds that can be absorbed by plants. Because the soil is rich in potassium bacteria and phosphorus bacteria, it is help to the soil minerals in the release of P and K, thus providing them to the growth and development of the plant [18]. From the previous studies, it can be seen that phosphate solubilization of phosphate-solubilizing bacteria is one of the important mechanisms to promote the growth of plants [19]. On the other hand, microbes can change the air in the soil, and convert the inert nitrogen into the ionic state so that the plant can absorb directly, so as to ensure the plant nitrogen nutrition. There are many kinds of nitrogen fixing bacteria, with more than 100 species, including in situ, symbiotic and combined nitrogen fixing bacteria, which play a very important role in promoting the growth of plants.
Some fungi and plant roots can form a kind of combination of mycorrhizal fungi, which can improve the ability of plant disease resistance and the ability to adapt to the soil environment. The relationship between fungi and plant roots is a kind of mutual promotion, which is not only conducive to the growth of plants, but also conducive to the survival of fungi [20]. With the extensive and long-term use of chemical fertilizers and pesticides in agricultural production, there are a lot of pollutants in the soil which are difficult to be degraded. Microorganisms can effectively degrade inorganic pollutants and organic pollutants which are harmful to plants [21]. Take heavy metal pollution as an example. Microorganisms can transform it into a low toxic or non-toxic form, to create a good environment for the growth of plants. In the activities, microorganisms can form a large number of humus acid, which also contributes to the growth of plants. Some soil microorganisms can also secrete some plant hormones in the late stage of their life cycle. These plant hormones can stimulate the development of plant roots, so as to increase the absorption of nutrients in plants [22]. When the plant rhizosphere and soil microbes form an ecosystem, it can form a physical barrier around the root system, which can effectively reduce the pathogen and pest invasion. Therefore, how to optimize the soil beneficial microorganisms, for the growth of plants has a very important significance.
Experimental Materials and Methods
Experimental materials
The soil sample used for the planting of the kiwifruit is from a crop planting base, which is purple soil. The soil is weakly alkaline, and the soil sampling is stored in the 4°C environment after a full mixing. After the laboratory test, the physical and chemical properties of the soil are shown in Table 1. The seedlings of the kiwifruit used in this experiment are provided by one Kiwi planting base in Guiyang. The growth cycle of all seedlings is basically the same, and the random selection is carried out in the laboratory.
| Name | PH value | Total N/(g/kg) | Total P/(g/kg) | Total K/(g/kg) | Organic matter/(g/kg) | Alkali solution nitrogen/(mg/kg) |
| Numerical value | 7.3 | 8.13 | 0.94 | 0.51 | 16.4 | 42.9 |
Table 1: The physical and chemical properties of the soil used in the experiment.
Nitrogen fixing bacteria, phosphate dissolving bacteria and potassium-solubilizing bacteria compose the beneficial bacteria used for the optimization of soil microbial bacteria. On the one hand, these three kinds of colonies have a positive effect on the growth of the kiwifruit. On the other hand, the three strains have higher affinity with purple soil samples. The strain is provided by an agricultural research institute, and the content of each strain is the experimental data. Because it has not yet put into practical production applications, it is not available to provide specific data. After culture expansion of nitrogen fixing bacteria, phosphate dissolving bacteria and potassium-solubilizing bacteria, they are uniformly mixed with the sterilized peat, and the mixed concentration is about 108 CFU/g. The physical and chemical properties of peat are shown in Table 2.
| Name | PH value | Total N/(g/kg) | Total P/(g/kg) | Total K/(g/kg) | Organic matter/(g/kg) | Alkali solution nitrogen/(mg/kg) |
| Numerical value | 4.9 | 10.4 | 2.18 | 6.11 | 451.28 | 792 |
Table 2: Physical and chemical properties of peat by experiment.
Experimental methods
The experiment is a controlled experiment. The experimental group is added with probiotics to improve soil microbial environment, while the control group is not added. Through the 90 d’s growth and development of kiwifruit, the growth of the seedlings is analyzed, so as to study the effect of soil beneficial microorganisms on the growth of kiwifruit. And the comprehensive analysis is made from three aspects of the plant growth situation, the disease resistance ability and the nutrient content.
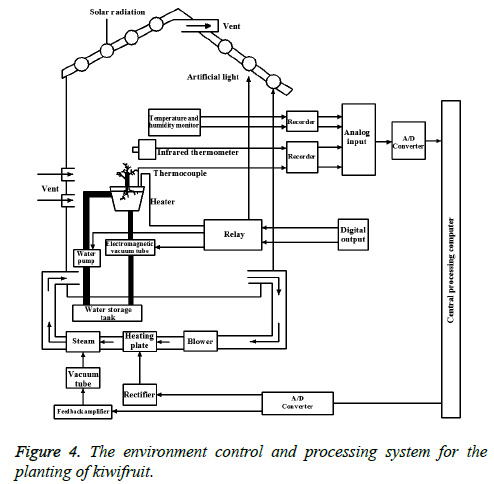
Take a large flower pot experiment with soil, and the soil is about 1.5 kg/pot. The soil should firstly use fertilizer, which is added for once. The experimental group is added by the peat with the uniform mixture of nitrogen fixing bacteria, dissolved phosphorus bacteria and potassium-solubilizing bacteria, with the dosage of 30 mg/kg, while the control group is added with the same amount of peat. The experiment group and the control group are marked with the flower pots, which are convenient for observation and data statistics. After adding fertilizers for 10d, the seedlings of kiwifruit are transplanted. After transplanting, the root of the kiwifruit is provided with the equal amount of water. The pots of the control group and the experimental group are randomly placed, and light, water volume, humidity and other external growth environment is strived to be the same. In order to better control the growth environment, computer processing control system is used to control lighting, water supply, ventilation, and the monitoring of the planting environment, including temperature, humidity and so on. The specific control system is shown in Figure 4.
Firstly, the amount of soil microorganisms should be determined to analyze the microbial community after adding the beneficial bacteria in experimental group. The determination of microbial number is mainly for bacteria, phosphate solubilizing bacteria, potassium-solubilizing bacteria and nitrogen fixing bacteria. The soil samples collected by five sampling points are stored in a refrigerator in 4°C after uniform mixing. Beef extract peptone medium is used to culture bacteria at 37°C for 24 h. Ashby nitrogen free medium is used to culture nitrogen fixing bacteria at 28°C for 5 d. Potassium-solubilizing bacteria medium is chosen to culture potassium-solubilizing bacteria at 28°C for 3 d. PKO inorganic phosphorus medium is selected to culture phosphate dissolving bacteria at 28°C for 3 d.
The second is the comparative analysis of the growth of kiwifruit. After 90 d, the plant height, leaf area and ground diameter of both the control group and the experimental group are measured, respectively. Then the samples are sampled from the upper part and the underground part of the kiwifruit plant. After being fixed at 105°C for 10min, they are dried at 80°C. The dry weight of the plant aboveground and underground parts are determined. Using the plant leaf's MDA and the defense enzyme activity content as the standard in the study on resistance property of kiwifruit plant, the contents of PAL, POD and MDA are measured in the laboratory, and the comparative analysis of two groups of experiments are carried on. The contents of N, P and K are measured by the method of distillation, and the content of total phosphorus and total potassium are determined by using vanadium molybdenum yellow colorimetry and flame photometry. All data are recorded and analyzed using EXCEL and SPSS software for statistical analysis.
Results Analysis
Experimental results
The number of soil microorganisms is determined, including bacteria, nitrogen fixing bacteria, phosphate dissolving bacteria and potassium-solubilizing bacteria, and the specific number is shown in Table 3. It can be seen from Table 3, compared with the control group, the amount of microorganisms in the experiment group is significantly higher than that of the control group, and adding probiotics can effectively increase the number of kiwifruit root microorganism. Through comparative analysis, it can be found that the content of bacteria, nitrogen fixing bacteria, phosphate dissolving bacteria and potassium-solubilizing bacteria in the experimental group is, respectively, 9 times, 47.8 times, 9.1 times and 6.6 times higher than that of the control group.
| Grouping | Bacteria (× 106 cfu·g-1) | Nitrogen fixing bacteria (× 106 cfu·g-1) | Phosphate dissolving bacteria (× 106 cfu·g-1) | Potassiumbacteria (× 106 cfu·g-1) |
|---|---|---|---|---|
| Comparison group | 4.02 ± 2.19 | 0.56 ± 0.24 | 3.62 ± 2.17 | 8.06 ± 4.25 |
| Experience group | 40.3 ± 5.03 | 27.3 ± 10.85 | 36.7 ± 9.05 | 61.4 ± 5.58 |
Table 3: Soil microbial biomass determination.
Then the growth of the plant is observed and analyzed, including plant height, earth diameter, leaf area, dry weight on the ground and dry weight of the ground. The specific conditions of the plant are shown in Table 4. It can be seen from Table 4, the growth of kiwifruit in the experimental group is better than that of the control group, and the soil added with bacteria is helpful for kiwifruit’s growth. Compared with the control group, the plant height, earth diameter, leaf area, dry weight on the ground and the dry weight of the underground part in the experimental group is, respectively, 120.1%, 50%, 6.2%, 200%, 166.7% higher.
| Grouping | Plant height/(cm) | Earth diameter/(mm) | Leaf area/(cm2) | Dry weight/(g) | Dry weight of underground/(g) |
|---|---|---|---|---|---|
| Comparison group | 9.1 ± 1.12 | 0.2 ± 0.02 | 77.23 ± 4.12 | 0.7 ± 0.23 | 0.3 ± 0.15 |
| Experience group | 20.1 ± 4.25 | 0.3 ± 0.04 | 82.07 ± 11.80 | 2.1 ± 0.74 | 0.8 ± 0.22 |
Table 4: Analysis and comparison of the growth of the seedlings of the kiwi fruit.
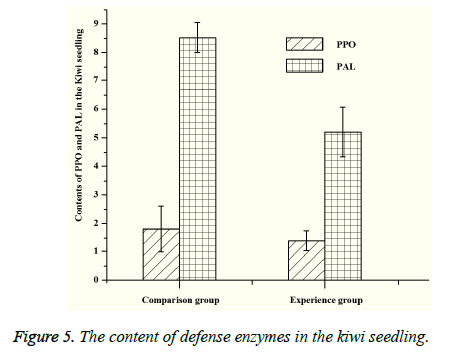
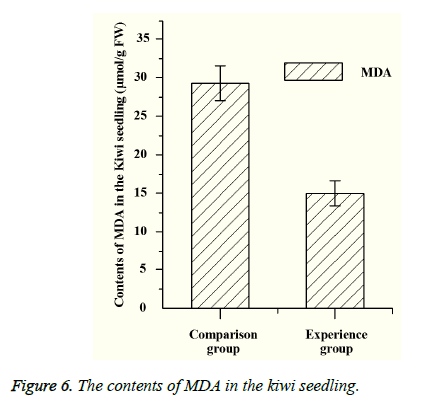
Both PPO and PAL are related to the metabolism of plants. PPO can catalyze the oxidation of polyphenols, which can make the plant tissue produce protective reaction. And PAL also has a certain relationship with plant disease resistance. The content of MDA represents the degree of lipid peroxidation, and the MDA’s content is negatively correlated with the plant resistance under the stress. By measuring the content of PPO and PAL (Figure 5), the content of PPO and PAL in the experimental group is lower than that of the control group, which indicates that the disease resistance of the experimental group is better than that of the control group. According to the measurement of malondialdehyde (MDA), it can be seen that the content of MDA in experimental group is significantly lower than that of the control group (Figure 6), which shows that the bacteria can increase the pathogen resistance of kiwifruit seedlings to a certain extent.
The contents of N, P, K in the ground and underground parts of the plant are shown in Table 5. It can be seen that the N, P, K nutrient content of the experimental group is higher than that of the control group. Among them, the content of N, P, and K in the upper part of the experimental group is, respectively, 12.0%, 19.4%, 52.2% higher than that of the control group, and the content of N, P, and K underground part of the experimental group is, respectively, 10.8%, 28.3%, and 21.9% higher than that of the control group. This fully shows that the improved soil microorganism with beneficial bacteria can improve the absorption of nitrogen, phosphorus and potassium, which is conducive to promoting the growth and development of kiwifruit.
| Grouping | Above ground part | Underground part | ||||
|---|---|---|---|---|---|---|
| N (g.kg-1) | P (g.kg-1) | K (g.kg-1) | N (g.kg-1) | P (g.kg-1) | K (g.kg-1) | |
| Comparison group | 8.18 ± 0.19 | 0.62 ± 0.02 | 11.3 ± 0.08 | 7.34 ± 0.62 | 0.53 ± 0.03 | 11.4 ± 0.63 |
| Experience group | 9.16 ± 0.27 | 0.74 ± 0.01 | 17.2 ± 0.63 | 8.13 ± 0.77 | 0.68 ± 0.01 | 13.9 ± 0.50 |
Table 5: The contents of N, P, K and underground parts in the seedlings of Chinese.
Discussion and Analysis
Using phosphate solubilizing bacteria, nitrogen fixing bacteria, and potassium-solubilizing bacteria to improve soil microbial community can effectively increase the number of beneficial microorganisms in soil, which illustrates the feasibility of artificial soil microbial optimization. As the experimental soil is rich in a variety of nutrients needed for growth of kiwifruit, both the kiwifruit of the experimental group and the control group can growth healthy. After adding beneficial bacteria, the growth of the experimental group is obviously superior to that of the control group. On the one hand, it shows the positive promoting effect of microorganism on the growth of kiwifruit. On the other hand, it also indicates that better growth of the kiwifruit can be achieved by optimizing the soil microbes.
Through the analysis of the experimental data, it can be seen that the effect of beneficial microorganisms on the growth of plants is embodied as follows: (1) The beneficial microbes have a direct influence on the growth of the seedlings of the kiwifruit, which can significantly improve the dry weight of the ground and the underground parts, and increase the plant height, the diameter and the leaf area. (2) The beneficial microbes can improve the ability of disease resistance of the plant, and reduce the incidence of the disease. (3) The increase in the number of beneficial microorganisms can improve the absorption of N, P and K nutrient elements. Therefore, in the production and cultivation of kiwifruit, according to different soil properties, a certain proportion can be added to increase the beneficial soil microbes, and optimize the soil microbial environment. So, it can improve the growth and development of the kiwifruit, reduce the occurrence of diseases, improve the yield of the kiwifruit, and thus meet the demand of the consumption of China.
Conclusion
By studying the relationship between the beneficial microbes and the growth of the kiwifruit, it is helpful to provide reference basis for the optimization of soil beneficial microorganisms used for the cultivation and cultivation of the kiwifruit. Using the control experiment, the changes of soil microbial quantity and the corresponding growth of the kiwifruit are analyzed. The experimental group is added with phosphate solubilizing bacteria, nitrogen fixing bacteria, and potassium-solubilizing bacteria, and it is cultivated for 90d experiment under the same favorable growth environment. Though the experimental results of the analysis, we can see that the contents of bacteria, phosphate solubilizing bacteria, nitrogen fixing bacteria, and potassium-solubilizing bacteria in the experimental group are significantly higher than those of beneficial microbes in the control group, in which the nitrogen fixing bacteria is 47.8 times higher. The height, earth diameter, leaf area, dry weight of ground and dry weight of underground of the kiwifruit in the experimental group are higher than those of the control group, and except for the high leaf area of 6.2%, the other parts are significantly increased.
The contents of PPO and PAL in the experimental group are significantly lower than those of the control group, and the MDA content is significantly higher than that of the control group, which shows that the beneficial microorganisms can significantly improve the disease resistance of the kiwifruit. The contents of N, P and K in kiwifruit are compared and analyzed. It can be seen that the contents of the ground and underground parts of the experimental group are higher than those of the control group, and the K content of the ground part is the most obvious, as high as 52.2%. Therefore, the optimized beneficial soil microbes contribute to kiwifruit growth and development, which can be used in cultivation. But because the growth lasts only for 90d in this experiment, it is necessary to analyze the effect of the beneficial microorganisms on the yield and quality of the kiwifruit.
References
- Wang C, Ai J, Qin H. First Report of Sclerotinia nivalis Causing Sclerotinia Rot on Hardy Kiwifruit (Actinidia arguta) in China. Plant Disease 2016.
- Müller K, Deurer M, Clothier BE. Eco-efficiency of organic and integrated kiwifruit production. Acta Horticulturae 2016: 447-454.
- Feng Y, Yang Q, Wang L. Survey of microbial contamination and characterization of Escherichia coli in kiwifruit orchards in Shaanxi, China, 2013. Foodborne Pathogens Disease 2015.
- Blatchford P, Stoklosinski H, Walton G. Kiwifruit fermentation drives positive gut microbial and metabolic changes irrespective of initial microbiota composition. Bioactive Carbohydrates Dietary Fibre 2015; 6: 37-45.
- Huang X, Liu S, Wang H, Hu Z, Li Z, You Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol Biochem 2014; 73: 42-48.
- García-Orenes F, Morugán-Coronado A, Zornoza R, Scow K. Changes in soil microbial community structure influenced by agricultural management practices in a Mediterranean agro-ecosystem. PloS one 2013; 8: e80522.
- D’Evoli L, Moscatello S, Lucarini M. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa, Planch. cv. Hayward) grown in Italy. J Food Composition Anal 2015; 37: 25-29.
- Femenia A, Sastre-Serrano G, Simal S. Effects of air-drying temperature on the cell walls of kiwifruit processed at different stages of ripening. Lebensmittel-Wissenschaft und-Technologie 2009; 42: 106-112.
- Yin XR, Allan A C, Xu Q. Differential expression of kiwifruit ERF, genes in response to postharvest abiotic stress. Postharvest Biol Technol 2015; 66: 1-7.
- Mishra S, Monro J. Kiwifruit remnants from digestion in vitro, have functional attributes of potential importance to health. Food Chem 2012; 135: 2188-2194.
- Matsumoto S, Obara T, Luh BS. Changes in Chemical Constituents of Kiwifruit During Post-Harvest Ripening. J Food Sci 2016; 48: 607-611.
- Vannini C, Domingo G, Marsoni M. Proteomic changes and molecular effects associated with Cr(III) and Cr(VI) treatments on germinating kiwifruit pollen. Phytochemistry 2011; 72: 1786-1795.
- Shiri MA, Ghasemnezhad M, Moghadam JF. Effect of CaCl2, Sprays at Different Fruit Development Stages on Postharvest Keeping Quality of “Hayward” Kiwifruit. J Food Process Preserv 2015; 40: 624-635.
- Henare SJ, Rutherfurd SM, Drummond LN. Digestible nutrients and available (ATP) energy contents of two varieties of kiwifruit (Actinidia deliciosa, and Actinidia chinensis). Food Chemistry 2012; 130: 67-72.
- Hill R, Stark C, Cummings N. Use of beneficial microorganisms and elicitors for control of pseudomonas syringae pv. Actinidiae in kiwifruit (Actinidia spp.). Acta Horticulturae 2015; 1095: 137-146.
- Fu Q, Gu J, Li Y. Analyses of microbial biomass and community diversity in kiwifruit orchard soils of different planting ages. Acta Ecologica Sinica 2015; 35: 22-28.
- Nardozza S, Boldingh H, Richardson A. Kiwifruit Xylem Sap: Composition and In Vitro Growth of a Virulent Strain of Pseudomonas syringae pv. actinidiae. Acta Horticulturae 2015: 123-128.
- Yang X , Chen Y. Identification of Pseudomonas syringae pv. actinidiae strains causing bacterial canker of kiwifruit in the Anhui Province of China, and determination of their streptomycin sensitivities. Genetics Mol Res Gmr 2015; 14: 8201-8210.
- Fraser LG, Datson PM, Tsang GK. Characterisation, evolutionary trends and mapping of putative resistance and defence genes in Actinidia, (kiwifruit). Tree Genetics Genomes 2015; 11: 1-15.
- Fan L, Zhou X, Li Y. The Influence of Effective Microorganisms on Microbes and Nutrients in Kiwifruit Planting Soil. Appl Sci 2016.
- Ran G. Colonization of Bacillus Strains Q13 and Q14 on the Kiwifruit Leaf Surface and Their Effects on the Leaf Microbial Flora. Biotechnol Bulletin 2012.
- Funayama M, Arakawa H, Yamamoto R. A New Microorganism Producing a Glucosyl Transfer Enzyme to Polyphenols. Biosci Biotechnol Biochem 2014; 58: 817-821.