ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Synthesis and anti-candidal activity of some new pyrazoline derivatives
1Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
2Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Mashooq Ahmad Bhat
Department of Pharmaceutical Chemistry
College of Pharmacy
King Saud University
Saudi Arabia
Accepted on December 6, 2016
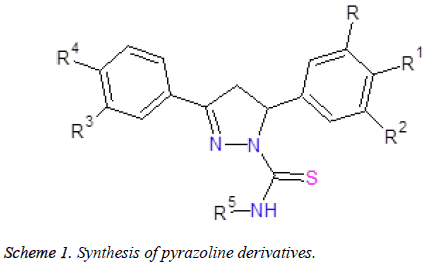
New pyrazoline derivatives were synthesized via the reaction of chalcones with cyclohexyl/phenyl thiosemicarbazide in presence of ethanol and acetic acid. All the compounds were confirmed by FT IR, 1H NMR, 13C NMR and MS spectral data. The compounds (1-11) were evaluated for antifungal activity against various strains of Candida species and compared with standard drug Itraconazole. MIC50 values were found to be within the range of 41.099-127.895 μg/ml and MIC90 values were found to be within the range of 62.121-240.955 μg/ml. Compound 7 was found to be most potent antifungal agent against Candida strains. It appears that para-methoxy substitution at one phenyl ring and meta-methoxy substitution at other phenyl ring of pyrazoline moiety made a significant contribution to the anti- Candidal activity in this series of pyrazolines.
Keywords
Pyrazoline, Chalcones, Anti-candidal activity, Thiosemicarbazides.
Introduction
Invasive Fungal Infections (IFIs) are the opportunistic infections in patients especially with weak immune function [1]. Candida spp. is responsible for majority of these infections [2]. Azoles are currently used as antifungal agents. These drugs are acting by inhibiting cytochrome 450 dependent 14 α- demethylase. Imidazoles and triazoles are the commonly found moieties in azole drugs. Ketaconazole, an imidazole derivative, is used as antifungal agent. Pyrazoles are the structural isomers of imidazole and pyrazolines are the reduced forms of the pyrazoles. Pyrazoline derivatives have been reported to possess biological activities, which include anti-tumor [3,4], antiinflammatory [5-7], anti-parasitary [8], anticonvulsant [9], antimicrobial [10-14] and antifungal [15,16]. Method for the synthesis of pyrazoline compounds from α, β-unsaturated carbonyl compounds (chalcones) is by the cyclization with hydrazine hydrate/substituted hydrazine. Based on molecular modelling and docking studies, the possible target for antifungal activity of arylthiosemicarbazides is the Nmyristoyltransferase (NMT) enzyme. High electron density around sulphur atom and geometry of N-N-C (=S)-NH pharmacophore is responsible for ligand recognition [17]. Based on the literature survey and in continuation of our work on the synthesis of anti-Candidal agents [18,19], we present herein the syntheses of new pyrazolines which have embedded thiosemicarbazide pharmacophore and are found to possess an interesting anti-Candidal activity.
Material and Methods
Experimental
Chemistry: All the solvents were procured from Merck. The compounds were checked by TLC performed on silica gel G coated plates (Merck). Visualization of TLC spots was done in iodine chamber. The FT-IR spectra were recorded in KBr pellets on a (Spectrum BX) Perkin Elmer FT-IR spectrophotometer. Melting points were recorded on a Gallenkamp melting point apparatus, and are uncorrected. NMR Spectra were scanned in DMSO-d6 on a Bruker NMR spectrophotometer (500 MHz) for 1H and (125 MHz) for 13C at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia. The molecular masses of compounds were determined by UPLC/TQMS.
General procedure for the synthesis of chalcone thiosemicarbazones: A mixture of appropriate substituted acetophenones (10 mmol), substituted benzaldehydes (10 mmol) and 60% aqueous NaOH solution (10 ml) in ethanol (20 ml) was stirred at 0°C for 4 hours. The reaction mixture was kept at room temperature for 48 hours and then water (50 ml) was added, 2 N HCl (50 ml) was used for neutralization and the mixture was extracted with EtOAc (3 × 200 ml). The organic layer was separated, washed with water several times, dried over anhydrous MgSO4 and concentrated over under reduced pressure. It was purified by column chromatography eluted with mixtures of hexane/EtOAc (70/30) to give pure chalcone derivatives.
General procedure for synthesis of pyrazoline derivatives of chalcone: To a mixture Chalcone (0.05 mmol) and thiosemicarbazide (0.05 mmol) in ethanol (50 ml), glacial acetic acid (250 μl) was added as a catalyst and stirred at 80°C for 24 h. The reaction mixture was concentrated by rotavapour. Purification of compounds was done by column chromatography on silica gel with mixtures of hexane/EtOAc (70/30), to yield pure pyrazoline derivative.
5-[4-(dimethylamino) phenyl]-3-(4-methoxyphenyl)- N-cyclohexyl-4, 5-dihydro-1H-pyrazole-1- carbothioamide (1)
IR (KBr) cm-1: 3419 (NH str.), 2922 (=CH str.), 1603 (C=C str.), 1119 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.1-1.8 (10H, s, 5 × -CH2), 2.5 (1H, s, -CH), 2.9 (6H, s, 2 × - NCH3), 3.3 (3H, s, -OCH3), 4.1 (1H, dd, J=5.0 Hz, HA), 6.7 (1H, dd, J=5.0 Hz, HB), 6.71 (1H, dd, J=7.5 Hz, Hx), 7.5-7.9 (8H, m, Ar-H), 11.1 (1H, s, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 25.3, 25.6, 49.0, 52.7, 112.1, 121.7, 129.0, 143.6, 151.8, 175.4; MS (ESI) m/z 436 [M]+.
5-(4-hydroxyphenyl)-3-(4-methoxyphenyl)-Ncyclohexyl- 4, 5-dihydro-1H-pyrazole-1- carbothioamide (2)
IR (KBr) cm-1: 3413 (NH str.), 2929 (=CH str.), 1604 (C=C str.), 1035 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.1-1.7 (10H, s, 5 × -CH2), 2.5 (1H, s, -CH), 3.3 (1H, dd, J=7 Hz, HA), 3.8 (3H, s, -OCH3), 4.1 (1H, dd, J=7.5 Hz, HB), 5.8 (1H, dd, J=11.0 Hz, HX), 6.6-8.1 (8H, m, Ar-H), 9.3 (1H, s, - OH), 11.1 (1H, s, NH, D2O exchg. ); 13C NMR (DMSO-d6, 125 MHz) δ 15.6, 25.4, 32.5, 42.4, 49.0, 53.2, 55.8, 63.0, 65.4, 70.5, 114.3, 114.5, 115.5, 116.0, 116.2, 118.9, 123.9, 126.4, 127.1, 129.3, 131.1, 134.0, 144.0, 154.9, 156.7, 160.4, 161.6, 163.4, 174.2, 187.7; MS (ESI) m/z 409 [M]+.
5-(4-ethoxyphenyl)-3-(3-methoxyphenyl)-Ncyclohexyl- 4, 5-dihydro-1H-pyrazole-1- carbothioamide (3)
IR (KBr) cm-1: 3467 (NH str.), 2933 (=CH str.), 1599 (C=C str.), 1047 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.3 (3H, t, J=6 Hz, CH3), 1.3-1.8 (10H, s, 5 × -CH2), 2.5 (2H, s, - CH), 3.3 (1H, dd, J=7.5 Hz, HA) 3.8 (2H, q, J=5 Hz, -OCH2), 4.0 (3H, s, -OCH3), 4.2 (1H, dd, J=7.0 Hz, HB), 6.9 (1H, dd, J=7.5 Hz, HX), 7.6-8.1 (8H, m, Ar-H), 11.3 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 15.0, 25.4, 25.6, 26.8, 32.3, 49.0, 52.9, 55.9, 63.6, 114.2, 115.2, 119.8, 126.9, 127.7, 129.3, 130.9, 131.1, 132.2, 142.5, 143.6, 160.4, 160.9, 163.5, 175.9, 187.7; MS (ESI) m/z 437 [M]+.
5-(3, 4-dimethoxyphenyl)-3-(3-methoxyphenyl)-Ncyclohexyl- 4, 5-dihydro-1H-pyrazole-1- carbothioamide (4)
IR (KBr) cm-1: 3337 (NH str.), 2925 (=CH str.), 1601 (C=C str.), 1025 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.1-1.8 (10H, s, 5 × -CH2), 2.5 (2H, s, -CH), 3.1 (1H, dd, J=5.0 Hz, HA), 3.8 (9H, s, 3 × -OCH3), 4.1 (1H, dd, J=7.5 Hz, HB), 6.9 (1H, dd, J=11.5 Hz, HX), 7.0-7.8 (7H, m, Ar-H), 10.7 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 25.1, 25.3, 25.5, 49.0, 52.7, 53.6, 55.7, 55.9, 56.0, 56.2, 110.0, 111.1, 111.4, 112.0, 112.1, 114.2, 114.4, 115.6, 117.1, 120.0, 120.9, 122.2, 122.6, 124.1, 126.9, 129.1, 129.2, 129.8, 130.2, 131.0, 131.2, 137.4, 140.5, 144.1, 149.1, 149.3, 149.4, 150.0, 150.6, 151.4, 160.5, 175.5, 176.6; MS (ESI) m/z 453 [M]+.
3-(4-methoxyphenyl)-N-cyclohexyl-5-phenyl-4, 5- dihydro-1H-pyrazole-1-carbothioamide (5)
IR (KBr) cm-1: 3321 (NH str.), 2935 (=CH str.), 1605 (C=C str.), 1108 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.1-1.8 (10H, s, 5 × -CH2), 2.5 (H, s, -CH), 3.1 (1H, dd, J=5 Hz, HA), 3.8 (3H, s, -OCH3), 4.1 (1H, dd, J=5.0 Hz, HB), 6.5 (1H, dd, J=7.0 Hz, H), 6.9-8.3 (9H, m, Ar-H), 10.9 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 25.2, 25.3, 25.5, 32.1, 32.2, 49.0, 52.7, 53.7, 55.7, 112.4, 112.9, 113.0, 113.6, 114.2, 114.5, 115.6, 117.1, 122.2, 124.1, 126.8, 130.2, 130.9, 131.1, 144.6, 145.2, 150.3, 152.2, 160.5, 160.3, 176.0; MS (ESI) m/z 391 [M]+.
5-[4-(dimethylamino) phenyl]-3-(3-methoxyphenyl)- N-cyclohexyl-4, 5-dihydro-1H-pyrazole-1- carbothioamide (6)
IR (KBr) cm-1: 3352 (NH str.), 2927 (=CH str.), 1615 (C=C str.), 1037 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.1-1.9 (10H, s, 5 × -CH2), 2.5 (H, s, -CH), 2.8 (6H, s, 2 × - NCH3), 3.3 (1H, dd, J=5.0 Hz, HA), 3.8 (3H, s, -OCH3), 4.1 (1H, dd, J=5.0 Hz, HB), 5.8 (1H, dd, J=9.5 Hz, HX), 6.6-7.9 (8H, m, Ar-H), 11.0 (1H, NH, D2O exchg.); 13C NMR (DMSOd6, 125 MHz) δ 25.4, 25.6, 32.4, 42.4, 49.0, 53.3, 55.7, 63.2, 112.8, 116.5, 120.0, 126.7, 130.2, 131.2, 132.8, 149.9, 154.9, 159.9, 174.4; MS (ESI) m/z 436 [M]+.
3, 5-bis (4-methoxyphenyl)-N-phenyl-4, 5-dihydro-1Hpyrazole- 1-carbothioamide (7)
IR (KBr) cm-1: 3333 (NH str.), 2935 (=CH str.), 1594 (C=C str.), 1021 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 3.3 (1H, dd, J=5.0 Hz, HA), 3.8 (6H, s, 2 × -OCH3), 3.88 (1H, dd, J=5.0 Hz, HB), 6.5 (1H, dd, J=5.0 Hz, HX), 6.8-7.6 (13H, m, Ar-H), 11.2 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 31.1, 55.6, 55.7, 56.0, 112.1, 113.6, 113.8, 114.2, 114.5, 115.5, 115.6, 116.9, 119.8, 121.0, 122.0, 122.8, 125.3, 125.6, 125.7, 125.8, 128.5, 128.6, 128.8, 129.4, 129.5, 130.1, 130.3, 130.3, 131.2, 131.4, 137.2, 137.6, 137.7, 139.2, 139.4, 140.4, 143.6, 149.2, 160.0, 160.1, 160.7, 176.9, 206.9; MS (ESI) m/z 417 [M]+.
5-[4-(dimethylamino) phenyl]-3-(4-methoxyphenyl)- N-phenyl-4, 5-dihydro-1H-pyrazole-1-carbothioamide (8)
IR (KBr) cm-1: 3414 (NH str.), 2903 (=CH str.), 1601 (C=C str.), 1023 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 2.8 (6H, s, 2 × -NCH3), 2.9 (1H, dd, J=5.0 Hz, HA) 3.8 (3H, s, - OCH3), 3.85 (1H, dd, J=5.0 Hz, HB) 6.0 (1H, dd, J=5.0 Hz, HX), 6.7-8.0 (13H, m, Ar-H), 11.5 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 14.7, 26.8, 31.1, 55.6, 55.8, 55.9, 63.2, 111.1, 112.1, 112.2, 114.1, 114.3, 114.6, 116.4, 117.2, 121.5, 122.5, 123.7, 125.1, 125.5, 125.7, 125.8, 126.0, 126.8, 128.4, 128.5, 128.8, 129.3, 129.5, 129.6, 130.2, 130.7, 130.9, 131.0, 131.5, 139.6, 140.0, 144.6, 144.8, 149.5, 150.0, 152.0, 152.3, 156.0, 161.8, 163.2, 163.5, 173.5; MS (ESI) m/z 429 [M-1]+.
5-(4-ethoxyphenyl)-3-(3-methoxyphenyl)-N-phenyl-4, 5-dihydro-1H-pyrazole-1-carbothioamide (9)
IR (KBr) cm-1: 3325 (NH str.), 2927 (=CH str.), 1598 (C=C str.), 1035 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.3 (3H, t, J=4.5 Hz, CH3), 2.5 (2H, q, CH2), 3.1 (1H, dd, J=5.0 Hz, HA), 3.83 (3H, s, -OCH3), 3.9 (1H, dd, J=6.5 Hz, HB), 6.0 (1H, dd, J=5.0 Hz, HX), 6.8-7.5 (13H, m, Ar-H), 10.1 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 15.1, 31.1, 42.6, 49.0, 55.8, 63.3, 63.4, 112.5, 114.8, 117.3, 120.5, 125.4, 126.0, 127.2, 128.4, 130.2, 132.6, 135.0, 140.0, 155.8, 158.0, 159.9, 174.2, 206.9; MS (ESI) m/z 431 [M]+.
5-(3, 4-dimethoxyphenyl)-3-(3-methoxyphenyl)-Nphenyl- 4, 5-dihydro-1H-pyrazole-1-carbothioamide (10)
IR (KBr) cm-1: 3314 (NH str.), 2949 (=CH str.), 1598 (C=C str.), 1019 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 3.1 (1H, dd, J=5.0 Hz, HA), 3.7 (9H, s, 3 × -OCH3), 3.8 (1H, dd, J=6.5 Hz, HB), 6.7 (1H, dd, J=5.0 Hz, HX), 6.9-7.6 (12H, m, Ar-H), 11.1 (1H, NH, D2O exchg.); 13C NMR (DMSO-d6, 125 MHz) δ 31.1, 49.0, 55.7, 55.8, 55.9, 56.0, 56.2, 110.1, 111.2, 111.5, 112.1, 112.2, 114.1, 114.4, 115.6, 117.1, 121.0, 122.2, 122.7, 124.1, 125.3, 125.6, 125.8, 126.9, 128.5, 128.6, 128.9, 129.1, 129.2, 129.6, 130.0, 131.2, 137.7, 139.2, 139.4, 140.9, 144.1, 149.3, 149.4, 149.5, 150.0, 150.1, 150.7, 160.6, 176.7, 187.8, 207.0; MS (ESI) m/z 445 [M-2]+.
5-(3, 4-dimethoxyphenyl)-3-(4-methoxyphenyl)-Ncyclohexyl- 4, 5-dihydro-1H-pyrazole-1- carbothioamide (11)
IR (KBr) cm-1: 3314 (NH str.), 2949 (=CH str.), 1598 (C=C str.), 1019 (C=S str.); 1H NMR (DMSO-d6, 500 MHz) δ 1.2-1.8 (10H, s, 5 × -CH2), 2.3 (1H, s, CH), 3.1 (1H, dd, J=5.0 Hz, HA) 3.8 (9H, s, 3 × -OCH3), 4.2 (1H, dd, J=5.0 Hz, HB), 5.9 (1H, dd, J=4.5 Hz, HX), 6.6-8.0 (7H, m, Ar-H), 11.2 (1H, NH, D2O exchg.); MS (ESI) m/z 447 [M]+.
Anti-Candidal activity
Microorganisms: Candida albicans (ATCC® 90028™) strains were procured from American Type Culture Collection (ATCC) and another clinical strain of Candida was collected from King Khalid University Hospital, Riyadh. Both the strains were maintained on Mueller Hinton Agar plates during the experiment.
Experimental protocol: The log phase growth of Candida was transferred to Mueller Hinton Broth for incubation at 37°C. The turbidity of growth was adjusted to 0.5 McFarland turbidity standards. This microbial concentration was used for further experiment.
Initial screening of compounds: The compounds were dissolved in DMSO and were tested against Candida strains using disc diffusion method. The Mueller Hinton Agar was inoculated with test Candida albicans using a sterile disposable swab to make a lawn. The filter paper disks were placed on the lawn and added with 20 μL of each 500 μg/ml adjusted compounds. The plates were incubated at 37°C for 24 hours. Next day the compound showing zone of inhibition were selected for broth microdilution based MIC determination.
Minimum Inhibitory Concentration determination: The compounds were dissolved in Mueller Hinton Broth with DMSO as co-solvent. The 250 μg/ml highest concentration of each compound was taken in 96 well microtiter plate and serially diluted half fold to achieve 250, 125, 62.5, 31.25, 15.625, 7.81, 3.90, and 0 μg/ml concentration of compounds respectively. The 100 μL McFarland standard adjusted culture was added and plates were incubated at 37°C for overnight incubation. The growth was recorded by measurement of OD570 through Biotech Synergy HT plate reader. The percentage survival of Candida was plotted against compound concentration and minimum inhibitory concentration was determined for 50 and 90% inhibition while assuming 100% growth in media without any inhibitory compounds. Itraconazole was used as positive control while DMSO was used as internal standard.
Results and Discussion
The synthesis of pyrazoline derivatives (1-11) was carried out in two step reaction as outlined in (Scheme 1). These compounds were synthesized from the reaction of chalcones and cyclohexyl/phenyl thiosemicarbazides. Chalcones and thiosemicarbazides were reacted in a mixture of ethanol and acetic acid at 80°C for 24 hours. Compounds were obtained with yields of 60-70% (Table 1). Elemental analysis and TLC was used to check the purity of compounds. Compounds (1-11) were confirmed by FT IR, 1H NMR, 13C NMR and mass spectroscopy. All the compounds gave satisfactory analytical and spectral data.
| Compd. | R | R1 | R2 | R3 | R4 | R5 | Yield (%) | Mp (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | H | N (CH3 ) | H | H | OCH3 | C6H11 | 70 | 163-165 |
| 2 | H | OH | H | H | OCH3 | C6H11 | 60 | 123-125 |
| 3 | H | OC2H5 | H | OCH3 | H | C6H11 | 65 | 120-122 |
| 4 | OCH3 | OCH3 | H | OCH3 | H | C6H11 | 60 | 173-175 |
| 5 | H | H | H | H | OCH3 | C6H11 | 70 | 198-200 |
| 6 | H | N (CH3 ) | H | OCH3 | H | C6H11 | 65 | 185-187 |
| 7 | OCH3 | H | H | H | OCH3 | C6H5 | 70 | 123-125 |
| 8 | H | N (CH3 ) | H | H | OCH3 | C6H5 | 60 | 138-140 |
| 9 | H | OC2H5 | H | OCH3 | H | C6H5 | 60 | 118-120 |
| 10 | OCH3 | OCH3 | H | OCH3 | H | C6H5 | 70 | 140-142 |
| 11 | OCH3 | OCH3 | H | H | OCH3 | C6H11 | 70 | 138-140 |
Table 1. Physical data of synthesized compounds (1-11).
In the FT IR spectra of compounds (1-11), band in the region of 3467-3314 cm-1 was observed due to NH stretching vibrations. The band of =CH, C=C and C=S stretching vibrations of the compounds appeared in the region of 2949-2903 cm-1, 1605-1598 cm-1 and 1119-1019 cm-1 respectively. In the 1H NMR spectra of compounds (1-11), protons of CH2 group of pyrazoline ring resonated at δ=2.9-4.1 and 3.8-6.7 ppm as pair of doublets of doublets. The CH proton appeared at δ=5.8-6.7 ppm as doublet of doublets due to vicinal coupling of CH2 group of pyrazoline ring (JAB=5.0-7.0 Hz, JAX=5.0-7.5 Hz, JBX=4.5-11.5 Hz). All other aromatic and aliphatic protons were observed at expected positions. The mass spectral data were also consistent with expected structures. Elemental analysis of all the compounds gave satisfactory results.
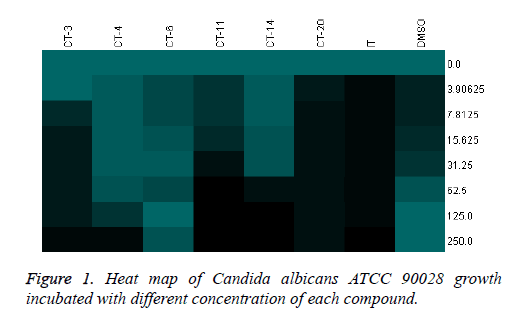
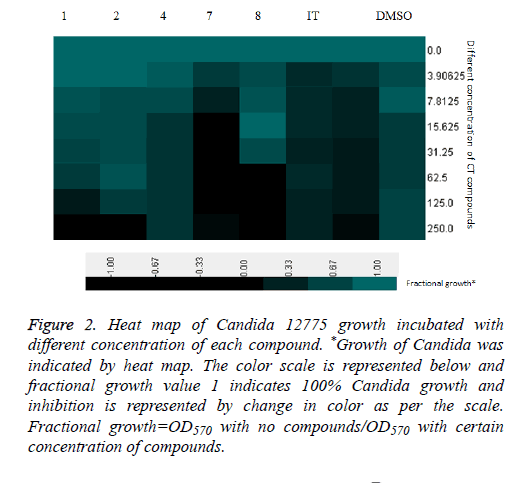
All the compounds of pyrazoline series (1-11) were screened for anti-Candidal activity by disc diffusion method. Candida 12775 and Candida albicans ATCC 90028 strains were used for screening of the compounds (1-11). MIC50 and MIC90 were recorded as minimum concentration of compound that inhibited 50% and 90% growth of the tested organism respectively. Compounds showing significant zone of inhibition were selected for calculation of MIC50 and MIC90 values. Compounds 1, 2, 4, 7 and 8 were selected for MIC50 and MIC90 determination. Itraconazole was used as positive control while DMSO was used as internal standard (Table 2). MIC50 values were found to be within the range of 41.099-127.895 μg/ml and MIC90 values were found to be within the range of 62.121-240.955 μg/ml. Comparing MIC50 values with itraconazole, all the five compounds were effective against Candida albicans ATCC 90028 and Candida 12775, especially compound 7 showed strong activity, similar level to the activity of itraconazole. MIC90 results of compounds also presented compound 7 as highly active against Candida albicans ATCC 90028 and Candida 12775. Heat map of Candida albicans ATCC 90028 growths incubated with different concentration of each compound (Figure 1) and heat map of Candida 12775 growth incubated with different concentration of each compound (Figure 2). The heat maps represent growth of respective Candida strains with certain concentration of each compound. The 100% growth which was considered as growth without addition of any inhibitory compounds was shown in green color while no growth is represented as black color. The respective color range and their percentage growth are given with the heat maps. The zero concentration of compound is showing same growth due to no inhibition, while further increase in compound concentrations led to change in growth sometime complete inhibition of growth (indicated by black color). The same graph of growth was used to calculate MIC50 and MIC90 of individual compound. This heat map gives better representation of growth inhibition of Candida strain with different concentration of compounds in comparison to simple MIC determination.
Figure 2: Heat map of Candida 12775 growth incubated with different concentration of each compound. *Growth of Candida was indicated by heat map. The color scale is represented below and fractional growth value 1 indicates 100% Candida growth and inhibition is represented by change in color as per the scale. Fractional growth=OD570 with no compounds/OD570 with certain concentration of compounds.
| Test compounds | Candida 12775 | Candida albicans ATCC 90028 | ||
|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | |
| 1 | 91.815 | 185.107 | 52.05 | 108.818 |
| 2 | 108.26 | 203.532 | 127.895 | 240.955 |
| 4 | 97.84 | 187.488 | 58.0145 | -- |
| 7 | 41.099 | 62.121 | 31.67 | 94.742 |
| 8 | 50.17 | 89.17 | 67.935 | 131.083 |
| Itraconazole | 38.18 | 111.204 | 31.634 | 73.63 |
| DMSO | 105.64 | 191.42 | -- | -- |
*--MIC was not able to determine as growth observed was variable due to either no inhibition (In case of DMSO with Candida albicans ATCC 90028) or due to color of compounds.
Table 2. MIC50 and MIC90 values of selected pyrazoline compounds against Candida.
Conclusion
Considering all the results obtained from the anti-candidal screening, in comparison with the reference drug itraconazole, it can be concluded that Compound 7 was found to be most potent compound of the series. The heat map represent that low concentration of this compound is sufficient to inhibit growth of Candida as indicated by black color. Based on the evaluation of compounds for anti-Candidal activity, it appears that para-methoxy substitution at one phenyl ring and metamethoxy substitution at other phenyl ring of pyrazoline moiety made a significant contribution to the anti-Candidal activity in this series of pyrazolines. Substitutional changes in cyclohexyl/ phenyl rings on the basic structure did not affect the activity.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this work through Research Group no. (RG 1435-006).
References
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20: 133-163.
- Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 2008; 14: 5-24.
- Johnson M, Younglove B, Lee L, LeBlanc R, Holt H Jr. Design, synthesis, and biological testing of pyrazoline derivatives of combretastatin-A4. Bioorg Med Chem Lett 2007; 17: 5897-5901.
- Ratkovic Z, Juranic ZD, Stanojkovic T, Manojlovic D, Vukicevic RD, Radulovic N, Joksovic MD. Synthesis, characterization, electrochemical studies and antitumor activity of some new chalcone analogues containing ferrocenyl pyrazole moiety. Bioorg Chem 2010; 38: 26-32.
- Rafia B, Syed O, Shafiya Y, Hinna H, Alam MS, Mohammad S, Surender S, Kalim J. Synthesis of some new 1, 3, 5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg Med Chem Lett 2011; 21: 4301-4305.
- Sameena B, Kalim J, Shamim A, Rathish IG, Surender S, Alam MS. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur J Med Chem 2011; 46: 5763-5768.
- Rathish IG, Kalim J, Shamim A, Sameena B, Alam MS, Pillai KK, Surender S, Vivek B. Synthesis and anti-inflammatory activity of some new 1, 3, 5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 2009; 19: 255-258.
- Bhat AR, Athar F, Azam A. New derivatives of 3, 5-substituted-1, 4, 2-dioxazoles; synthesis and activity against Entamoeba histolytica. Eur J Med Chem 2009; 44: 926-936.
- Zuhal OH, Burak KBGX, Unsal C, Alisx A, Altan B. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 2007; 42: 373-379.
- Manna K, Agrawal YK. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity. Bioorg Med Chem Lett 2009; 19: 2688-2692.
- Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2 yl)-4, 5-dihydro-5-aryl-1-[4-(aryl)-1, 3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem 2009; 44: 2632-2635.
- El-Sayed WA, Nassar IF, Abdel-Rahman AA-H. C-Furyl glycosides, II: Synthesis and antimicrobial evaluation of C-furyl glycosides bearing pyrazolines, isoxazolines, and 5, 6-dihydropyrimidine-2 (1H)-thiones. Monatsh Chem 2009; 140: 365-370.
- Jadhav SB, Shastri RA, Gaikwad KV, Gaikwad SV. Synthesis and antimicrobial studies of some novel pyrazoline and isoxazoline derivatives. Eur J Chem 2009; 6: 183-188.
- Sakthinathan SP, Vanangamudi G, Thirunarayanan G. Synthesis, spectral studies and antimicrobial activities of some 2-naphthyl pyrazoline derivatives. Spectrochim Acta A 2012; 95: 693-700.
- Bekhit AA, Abdel-Aziem T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem 2004; 12: 1935-1945.
- Ali I, Wani WA, Haque A, Ahmad A, Saleem K, Manzoor N. Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its copper (II) and nickel (II) complexes with conventional antifungals. Microb Pathog 2012; 53: 66-73.
- Siwek A, Staczek P, Stefanska J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur J Med Chem 2011; 46: 5717-5726.
- Bhat MA, Khan AA, Khan S, Al-Dhfyan A. Synthesis of new [1, 2, 4] triazolo [3, 4-b] [1, 3, 4] thiadiazines and study of their anti-Candidal and cytotoxic activities. J Chem 2014; 2014: 1-7.
- Bhat MA, Khan AA, Khan S, Al-Omar MA, Parvez MK. Synthesis and anti-Candidal activity of N-(4-aryl/cyclohexyl)-2-(pyridine-4-yl carbonyl) hydrazinecarbothioamide. Bioorg Med Chem Lett 2014; 24: 1299-1302.