ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
Synthesis of 4-arylpolyhydroquinoline derivatives and evaluation of their anti-inflammatory on endometritis
1Department of Gynaecology and Obstetrics, People's Second Hospital of Liaocheng Affiliated to Taishan Medical University, Linqing 252601, PR China
2Department of Pathology, People's Second Hospital of Liaocheng Affiliated to Taishan Medical University, Linqing 252601, PR China
- *Corresponding Author:
- Ling-Ling Teng
Department of Gynaecology and Obstetrics
People's Second Hospital of Liaocheng
Taishan Medical University
PR China
Accepted date: March 24, 2016
Four novel 4-arylpolyhydroquinoline derivatives (1-4) were synthesized and characterized via IR, 1H NMR, HRMS, and single crystal X-ray crystallography. The experimental results of anti-inflammatory activity showed that compared with compounds 1 and 2, compounds 3 and 4 with electron-repelling groups in the phenyl ring exerted rather potent activities.
Keywords
4-Arylpolyhydroquinoline, Crystal, Anti-inflammatory.
Introduction
Endometritis is inflammation of the endometrium, the inner lining of the uterus. Pathologists have traditionally classified endometritis as either acute or chronic [1]. Acute endometritis is characterized by the presence of microabscesses or neutrophils within the endometrial glands, while chronic endometritis is distinguished by variable numbers of plasma cells within the endometrial stroma [2,3]. The most common cause of endometritis is infection. Symptoms include lower abdominal pain, fever and abnormal vaginal bleeding or discharge. Caesarean section, prolonged rupture of membranes and long labor with multiple vaginal examinations are important risk factors. Treatment is usually with broad-spectrum antibiotics [4].
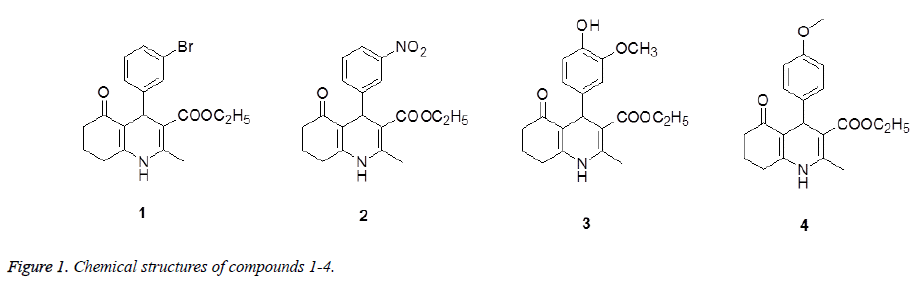
Quinoline derivatives are valuable intermediates in the preparations of a wide range of biologically active compounds such as anticancer agents, anti-inflammatory agent, and hedgehog antagonist, etc [5,6]. In addition, they also represent a class of functionalized and versatile building blocks, that is, these compounds can be converted into 4- arylpolyhydroquinoline derivatives [7,8]. With this in mind we report here the synthesis of four new 4- arylpolyhydroquinoline derivatives (Figure 1) along with their anti-inflammatory activity.
Materials and Methods
Apparatus and materials
IR spectra (400-4000 cm-1) were obtained using a Brucker Equinox-55 spectrophotometer. 1H NMR spectra were obtained using a Varian Inova-400 spectrometer (at 400 MHz). Mass spectra were obtained using a micrOTOF-Q II mass spectrometer. The melting points were taken on a XT-4 micro melting apparatus, and the thermometer was uncorrected.
Synthesis and characterization of compounds 1-4
Compounds 1-4 were synthesized according to a reported procedure [9]. A mixture of cyclohexane-1,3-dione (10 mmol), aromatic aldehydes (10 mmol), ammonium acetate (10 mmol) and ethyl acetoacetate (10 mmol) in ethanol (100mL) was refluxed for 2-3 h and then cooled to room temperature. After filtering the precipitates, they were sequentially washed with ice-cooled water and ethanol and then dried under a vacuum.
4-(3-Bromo-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline- 3-carboxylic acid ethyl ester (1): 235-236°C. Yield: 65.4 %. IR (KBr pellet cm-1): 3265, 1650, 1600 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.22 (s, 1H), 7.27-7.29 (m, 2H), 7.12-7.19 (m, 2H), 4.86 (s, 1H), 3.95-4.02 (m, 2H), 2.47-2.49 (m, 2H), 2.29 (s, 3H), 2.18-2.22 (m, 2H), 1.88-1.92 (m, 1H), 1.73-1.77 (m, 1H), 1.12 (s, 3H). HRMS (ESI+): m/z: calcd for C19H20BrNO3: 412.0519 [M+Na+]; found: 412.0522.
4-(3-Nitro-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline- 3-carboxylic acid ethyl ester (2): 225-226°C. Yield: 60.3 %. IR (KBr pellet cm-1): 3270, 1670, 1650 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.32 (s, 1H), 7.97-7.98 (d, 2H), 7.60-7.61 (d, 1H), 7.51-7.54 (t, 1H), 5.00 (s, 1H), 3.96-3.98 (t, 2H), 2.50-2.52 (m, 2H), 2.32 (s, 3H), 2.16-2.26 (m, 2H), 1.89-1.93 (m, 1H), 1.74-1.77 (m, 1H), 1.10-1.12 (t, 3H). HRMS (ESI+): m/z: calcd for C19H20N2O5: 379.1266 [M+Na+]; found: 379.1879.
4-(3-Methoxy-4-hydroxy-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8- hexahydro-quinoline-3-carboxylic acid ethyl ester (3): 230-231°C. Yield: 58.4 %. IR (KBr pellet cm-1): 3260, 1669, 1632 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.07 (s, 1H), 8.62 (s, 1H), 6.70-6.71 (d, 1H), 6.57-6.58 (d, 1H), 6.47-6.49 (q, 1H), 4.80 (s, 1H), 3.99-4.01 (q, 2H), 3.68 (s, 3H), 2.46-2.48 (m, 2H), 2.25 (s, 3H), 2.18-2.21 (m, 2H), 1.89-1.92 (m, 1H), 1.75-1.78 (m, 1H), 1.14-1.17 (t, 3H). HRMS (ESI+): m/z: calcd for C20H23NO5: 380.1477 [M+Na+]; found: 380.1460.
4-(4-Methoxy-phenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline- 3-carboxylic acid ethyl ester (2): 228-229°C. Yield: 68.2 %. IR (KBr pellet cm-1): 3212, 1678, 1600 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.09 (s, 1H), 7.03-7.04 (d, 2H), 6.73-6.74 (d, 2H), 4.82 (s, 1H), 3.97-3.98 (d, 2H), 3.67 (s, 3H), 2.45-2.48 (q, 2H), 2.27 (s, 3H), 2.14-2.20 (m, 2H), 1.87-1.91 (m, 1H), 1.73-1.75 (m, 1H), 1.12-1.14 (t, 3H). HRMS (ESI+): m/z: calcd for C20H23NO4: 364.1510 [M+Na+]; found: 364.1577.
Crystal structure determination
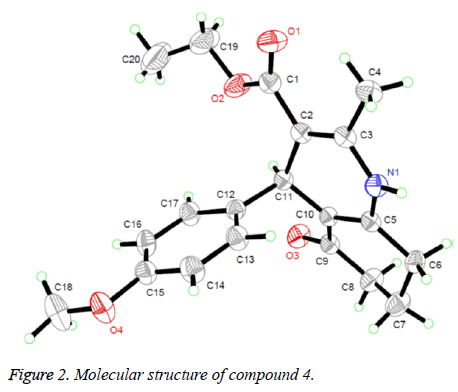
Suitable single crystals of compound 4 were obtained by evaporation of chloroform solution. The diffraction data were collected on a Bruker Smart Apex CCD area detector using a graphite monochromated Mo Kα radiation (λ=0.71073 Å) at room temperature. The structure was solved by using the program SHELXL-97 [10] and Fourier difference techniques, and refined by full-matrix least-squares method on F2. All hydrogen atoms were added theoretically. Crystallographic data for compound 4 are listed in Table 1.
| Formula | C20H23NO4 |
| Mr | 341.39 |
| Temperature/K | 293 (2) |
| Crystal system | Triclinic |
| Space group | Pī |
| a/Å | 7.2610 (19) |
| b/Å | 10.511 (3) |
| c/Å | 12.620 (3) |
| α/° | 74.305 (4) |
| β/° | 73.718 (4) |
| γ/° | 82.747 (5) |
| V/Å3 | 888.7 (4) |
| Z | 2 |
| Dcalc/g·cm-3 | 1.276 |
| μ(Mo Kα)/mm-1 | 0.089 |
| θ range/° | 1.73 to 25.00 |
| Reflections collected | 4575 |
| No. unique data [R(int)] | 3115 [0.0215] |
| No. data with I ≥ 2σ(I) | 2267 |
| R1 | 0.0574 |
| ωR2(all data) | 0.1762 |
| CCDC | 1469526 |
Table 1: Crystal data, data collection and structure refinement of compound 4.
Anti-inflammatory activity
Anti-inflammatory activity was measured by carrageenan induced paw edema as described by Winter et al. Drugs were given orally as solution is distilled water. The solution was so prepared that for every 100 g body weight of the animal 0.3 to 0.8 mL of the solution was administered depending on the dose. Groups of six rats were dosed orally with drug 1h before injection of 0.1 mL of a 1 % suspension of carrageenan in normal saline into the subplantar region of the right hind paw. Control animals (six) received carrageenan only. Oedema was measured 3h later plethysmographically. Mean increase in the paw volume and Standard Error of the Mean (SEM) for each group were calculated and the results were expressed as percent inhibition of oedema as compared to the control group. ED50 values were determined by semilogarithmic plot of the % inhibition versus log dose.
Results and Discussion
Molecular structure
The 1H NMR, IR and MS for the products are in good agreement with the title compounds. In the 1H NMR spectra of compounds 1-4, single peaks at δ 4.86, δ 5.00, δ 4.80 and δ 4.82 ppm characteristic of the CH group respectively. In order to confirm the configuration of the product, single crystals of compound 4 were cultured for X-ray diffraction analysis. The crystal structure of compound 4 was presented in figure 2. The six-membered ring containing nitrogen atom is nearly planar [maximum deviation=0.1734 Å] and the adjacent ketone ring adopts a flattened chair conformation [dihedral angle (C5-C6- C7-C8)=16.303°]. The nitrogenous heterocyclic is almost perpendicular to the benzene ring [dihedral angle=85.435°] and is almost coplanar with the mean plane of the ketone ring [dihedral angle=11.343°].
Biological activity
The anti-inflammatory activity of compounds 1-4 were evaluated by carrageenan induced paw edema in rats, and the ED50 values derived from the experimental data were summarized in table 2. As can be seen in table 2, there is great difference in the anti-inflammatory activity among the four compounds. Compared with compounds 1 and 2, compounds 3 and 4 showed more potent anti-inflammatory activity with ED50 value of 61.2 and 57.2 mg/kg, which is much lower than those of compounds 1 and 2.
| Compounds | Antiinflammatory activity (%) ± SME | |||
|---|---|---|---|---|
| Dose (mg/kg) | ED50 (mg/kg) | |||
| 25 | 50 | 100 | ||
| 1 | 0.7 ± 1.8 | 21.8 ± 1.3* | 29.0 ± 2.1 | 189.5 |
| 2 | 0.9 ± 1.9* | 20.2 ± 1.7 | 26.3 ± 2.6 | 193.5 |
| 3 | 20.1 ± 2.3 | 55.3 ± 2.1* | 66.2 ± 3.7 | 61.2 |
| 4 | 21.2 ± 2.1* | 59.9 ± 2.7 | 61.5 ± 3.5* | 57.2 |
| Anti-inflammatory activity by carrageenan induced paw edema in rats at the end of 3h. Drug was given orally as solution in distilled water (*p<0.05 Mann-Whitney test). Six rats were used for each test group and the control. | ||||
Table 2: Anti-inflammatory activity of compounds 1-4.
Conclusion
In conclusion, we synthesized four novel 4- arylpolyhydroquinoline derivatives and characterized them via IR, 1H NMR, HRMS, and single crystal X-ray crystallography. We have found that compared with compounds 1 and 2, compounds 3 and 4 exhibited the better anti-inflammatory efficiency with ED50 values of 61.2 and 57.2 mg/kg, possibly because of the electron-repelling group in the phenyl ring in the structures of compounds 3 and 4.
References
- Leblanc SJ, Duffield TF, Leslie KE. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 2002; 85: 2223-2236.
- Cohen CR, Manhart LE, Bukusi EA. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359: 765-766.
- Kurman RJ, Scully RE, Norris HJ. Trophoblastic pseudotumor of the uterus: an exaggerated form of "syncytial endometritis" simulating a malignant tumor. Cancer 1976; 38: 1214-1226.
- Kasimanickam R, Duffield TF, Foster RA. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004; 62: 9-23.
- Li J, Sui Y, Xin J. Synthesis of biscoumarin and dihydropyran derivatives with promising antitumor and antibacterial activities. Bioorg Med Chem Lett 2015; 25: 5520-5523.
- Li J, Lv CW, Li XJ. Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity. Molecules 2015; 20: 17469-17482.
- Li J, Hou Z, Chen GH. Synthesis, antibacterial activities, and theoretical studies of dicoumarols. Org Biomol Chem 2014; 12: 5528-5535.
- Li J, Zhou PP, Liu Z. Crystal Structure, Photoluminescence, and Theoretical Studies of Dihydropyran Derivatives. Chinese J Struct Chem 2015; 34: 319-323.
- Khan AT, Lal M, Ali S, Khan MM. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett 2011; 52: 5327-5332.
- Sheldrick GM. SHELXL-97, Program for Solution Crystal Structure and Refinement, University of Göttingen: Göttingen, Germany, 1997.