ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
Synthesis of some sulfonamide incorporating enaminone, quinolone moieties and thiazoloquinazoline derivative induce the cytoprotective enzyme NAD(P) H: Quinone Oxidoreductase 1.
Mostafa M Ghorab1,2*, Mansour S Alsaid1, Abdelaaty A Shahat1,3
1Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
2Department of Drug Radiation Research, National Center for Radiation Research and Technology, Atomic Energy Authority, Nasr City, Cairo, Egypt
3Phytochemistry Department, National Research Centre, 12311 Dokki, Cairo, Egypt
- *Corresponding Author:
- Mostafa M Ghorab
Department of Pharmacognosy
College of Pharmacy
King Saud University
Saudi Arabia
Accepted date: February 25, 2016
Sulfonamide resonant biologically active enaminone derivatives 3, 5, and quinolone derivative 8 have been created. Correspondingly, the thiazoloquinazoline derivative 13 was acquired in decent yield via reaction of 2-isothiocyanate derivative 9 with the L-nor ephedrine 10. The assemblies of the prepared amalgams stood established by microanalysis, IR, 1H-NMR, 13C-NMR and mass spectral information. Furthermore amalgams 3 was proved by X-ray crystallographic analysis. The NQO1 inducer activity of the synthesized compounds was assessed by means of a measurable bioassay in Hepa1c1c7 murine hepatoma cells. The thiazoloquinazoline (13) exhibited remarkable activity. Besides, incorporating the thiazol moiety within a heterocyclic ring system (quinazoline) increases the inducer potency. On the other hand, the enaminone derivatives (3 and 5) and quinoline (8) showed weak activity.
Keywords
NQO1, Electrophilicity cytoprotection, Enaminones, Quinolone, Quinazoline.
Introduction
Chemotherapy is the mainstay for cancer conduct, the custom of obtainable chemotherapeutics is recurrently inadequate in arrears to obnoxious sideways possessions [1]. Midst the heterocyclic orderings, particularly persons encompassing enaminone and pyridine ring are allied with diverse pharmacological assets for example cytoprotection [2,3], antibacterial [4-6], anticonvulsant [7], antiviral [8], anti-HIV [9], antifungal and antimycobacterial events [10]. Freshly substantial courtesy has been keen to the construction of new derivatives of quinoline on the justification of their stated biological activities [11-18]. From the prose review, numerous methods have been pronounced for the elaboration of substituted quinolones [19-21], which as a period have been testified to have anticancer and antileukemic activity. Quinolines were institute to own numerous pharmacological belongings, counting uncontaminated [22-24] besides antitumor [25-31] deeds. Likewise, the chemistry of quinazoline and fused quinazoline spinoffs has been of cumulative curiosity, subsequently numerous of these compounds demonstrated quite a few biotic accomplishments too beneficial request as per antitumor [32-40], besides antiseptic go-betweens [41]. Alternatively, amongst the extensive assortment of compounds experienced by way of antitumor go-betweens, sulfonamides have fascinated prodigious courtesy, as countless sulfonamide offshoots were testified to have thought-provoking antitumor bustle [42-46]. Quite a lot of devices have been stated for the anticancer movement of the sulfonamide composites then the maximum protuberant of these mechanisms was concluded the reserve of the carbonic anhydrase isozymes [47-50]. The appliance of lump reserve by sulfonamide Carbonic Anhydrase (CA) inhibitor was optional by Boyle and Chegwidden [51], that these compounds possibly will diminish the endowment of bicarbonate for the amalgamation of nucleotides and erstwhile cell components for instance membrane lipids. In persistence of our exertion it give the impression of curiosity to manufacture roughly innovative 4-(quinoline-1-yl)- benzenesulfonamide and pyrimido[4,5-b] (quinoline-10-yl) benzenesulfonamide spinoffs, manner hypothetically vigorous lateral manacles, for instance cyano [32], ureido, thioureido [34] and thione [39] as per equivalents of multifarious E7070 [50] (Figure 1), to be assessed as probable cytoprotective mediators. In this effort we synthesized the enaminone, quioloine and the fused tricyclic thiazoloquinazoline derivatives brilliant with improved electron kinship for superior biological interactions by mono, bicyclic and tricyclicfused heterocyclic systems to create compounds 3, 5, 8 and 13 to study their conceivable role in persuading the cytoprotective enzyme NQO1.
Experimental
Melting points (°C, uncorrected) were strong-minded in uncluttered capillaries on a Gallenkemp melting point gadget (SanyoGallenkemp, Southborough, UK). Pre-caked silica gel saucers (silica gel 0.25 mm, 60 G F 254; Merck, Germany) were castoff for thin layer chromatography, dichloromethane/ methanol (9.5: 0.5 mL) blend was cast-off as a emergent solvent system. IR spectra were logged in KBr discs via IR Shimadzu spectrometer (Shimadzu, Tokyo, Japan). NMR spectra in (DMSO-d6) were chronicled on Bruker Ac-500 ultra-shield NMR spectrometer (Bruker, Flawil, Switzerland, δppm) at 500 MHz, consuming TMS as internal orthodox. Elemental analyses were achieved on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). Entirely compounds were within ± 0.4 % of the hypothetical morals.
Results
4-(5, 5-Dimethyl-3-oxocyclohex-1-enylamino) benzenesulfonamide (3)
A mixture of 5,5-dimethyl-cyclohexane-1,3-dione 1 (1.40 g, 0.01 mol) and sulfanilamide 2 (1.72 g, 0.01 mol) in absolute ethanol (15 ml) was refluxed for 3 h. The reaction mixture was cooled and then poured onto cold water, the obtained solid was recrystallized from ethanol to give 3 [52]. Yield, 81%; m.p. 235-237 °C; IR, cm-1: 3478, 3314, (NH, NH2), 3055 (CH arom.), 2956, 2840 (CH aliph.), 1630 (C=O), 1324, 1150 (SO2). 1H- NMR (DMSO-d6) δ:1.1 [s, 6H, 3CH3], 2.1- 2.4 [2s, 4H, 2CH2], 5.3 [s, 2h, NH2], 5.5 [s, 1H, CH], 7.2, 7.7 [2d, 4H, AB-system Ar-H], 11.1 [s, 1H, NH, D2O-exchangeable]. MS, m/z (%): 294 [M+] (26.11%), 130 (100%). Anal. Calcd. For C14H18N2O3S (294): C, 57.12; H, 6.16; N, 9.52. Found: C, 57.50; H, 6.45; N, 9.18.
Oxocyclohexenylamino)benzenesulfonamide (5)
A mixture of 1,3-cyclohexanedione 4 (1.12 g, 0.01 mol) and sulfanilamide 2 (1.72 g, 0.01 mol) in ethanol (30 mL) was refluxed for 5 h. The reaction mixture was cooled and then poured onto cold water, the obtained solid was crystallized from ethanol to give 5 [53]: Yield, 88%; m.p. 236-238°C; IR, cm-1: 3354, 3263, 2210 (NH, NH2), 3032 (CH arom.), 2940, 2870 (CH aliph.), 1611 (C=O), 1360, 1184 (SO2). 1H-NMR (DMSO-d6) δ: 1.0-2.2 [m, 6H, 3CH2], 5.5 [s, 1H, CH], 7.0-7.8 [m, 6H, Ar-H SO2NH2], 9.0 [s, 1H, NH, D2O-exchangeable]. Anal. Calcd. For C12H14N2O3S: C, 54.12; H, 5.30; N, 10.52. Found: C,53.81; H, 5.62; N, 10.23.
4-[2-Amino-3-cyano-4-(2, 4-dichlorophenyl)-5-oxo-5, 6, 7, 8-tetrahydro quinolin-1(4H)- yl]benzenesulfonamide (8)
Method A: A mixture of enaminone 5 (2.66 g, 0.01 mol) and 2-(2,4- dichlorobenzylidine) malononi-trile 6 (2.23 g, 0.01 mol) in ethanol (20 mL) containing 3 drops of triethy1amine was refluxed for 5 h. The reaction mixture was filtered while hot and the solid obtained was crystallized from dioxane to give 8 [53].
Method B: A solution of enaminone 5 (2.66 g, 0.01 mol), 2,4- dichlorobenzaldehyde (1.75 g, 0.01 mol), and malononitrile (0.66 g, 0.01 mol) in absolute ethanol (20 mL) containing three drops of trimethylamine was refluxed for 6 h. The obtained solid after concentration was filtered and crystalilized from ethanol to give 8.Yield, 90%; m.p. 291-293 °C;IR, cm-1: 3464, 3347 (NH2), 3064 (CH arom.), 2957, 2860 (CHaliph.), 2171 (CN), 1634 (C=O), 1374, 1189 (SO2), 706 (C-Cl). 1H- NMR (DMSO-d6) δ: 1.8-2.2 [m, 6H, 3CH2], 4.9 [s, 1H, CH], 5.4 [s, 2H, NH2, D2O-exchangable], 7.1-8.0 [m, 9H, Ar-H SO2NH2]. 13C- NMR(DMSO-d6) δ: 21.9, 27.8, 34.8, 37.6, 58.6, 113.4, 116.5, 118.3 (CN),128.2, 129.6, 130.9, 131.7, 132.8, 133.6, 136.7, 142.6, 145.9, 155.6,167.8, 197.5 (C=O). MS, m/z (%): 489 [M+] (1.8), 73 (100). Anal. Calcd. For C22H18Cl2N4O3S: C, 53.99; H, 3.71; N, 11.45. Found: C,54.33; H, 3.49; N, 11.10.
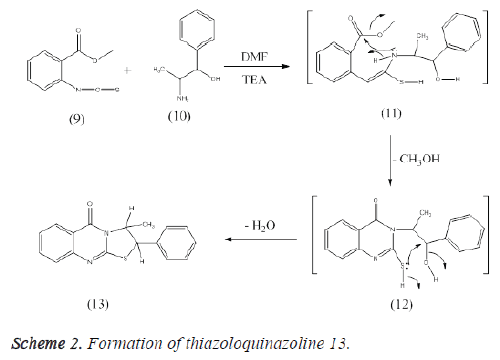
(1S, 2S)- 3-methyl-2-phenyl-2, 3- dihydrothiazolo [2, 3- b] quinazolin-5-one (13)
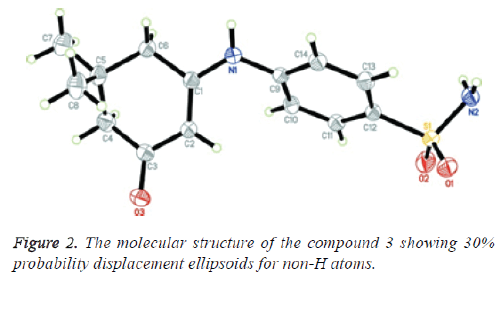
A mixture of 2-isothiocyanatobenzoate (1.93 g, 0.01 mole) and 2-amino-1-phenylpropan-1-ol (1.51 g, 0.01 mole) in dry dimethylformamide (30 ml) containing a catalytic amount of triethylamine was refluxed for 6 h. The solid obtained was recrystallized from ethanol to give (13) [54]. Yield, 97%; m.p. 158.5 °C; IR, cm-1: 3097 (CH arom.), 2975, 2848 (CHaliph.), 1686 (C=O), 1613 (C=N). 1H- NMR (DMSO-d6) δ: 1.3 [s, 3H, CH3], 4.3 [s, 2H, 2CH], 7.2-8.1 [m, 9H, Ar-H]. 13C- NMR (DMSO-d6) δ: 14.1 (CH3), 51.2 (S-CH), 64.8 (N-CH), 122.3, 127.6, 128.4 (2), 128.7, 128.9, 129.6 (2), 129.9, 131.8, 138.3, 141.2, 145.7, 157.8, 163.2 (C=O). MS, m/z (%): 294 [M+] (18.6), 78 (100). Anal. Calcd. For C17H14N2OS: C, 69.36; H, 4.79; N, 9.52. Found: C,69.08; H, 4.49; N, 9.77 (Figures 2 and 3).
Biological assay
The NQO1 inducer motion was robust -minded by earnings of a measurable microtiter plate assay [55]. Hepa1c1c7 cells were full-fledged in αMEM supplemented with 10% (v/v) fetal bovine serum that had been heat- and charcoal- inactivated. Cells were habitually upheld in a humidified atmosphere at 37°C, 5% CO2. For apiece experiment, cells (104 per well) were plated in 96-well plates. Subsequently 24 h, the cell culture medium was traded with fresh medium comprising enaminones, and the cells were grown for a further 48 h.
Eight repeats of 8 serial dilutions of each compound were rummage-sale. Compounds were primed as stock solutions in DMSO, and then diluted in the cell culture medium 1:1000. The final concentration of DMSO in the medium was maintained at 0.1% (v/v). At the end of the 48 h exposure period, cells were lysed for 30 min at 25°C in digitonin (0.1 g/L, pH 7.8). The precise activity of NQO1 was appraised in cell lysates by revenue of menadione as a substrate. Protein concentrations were strong-minded in apiece well by the BCA protein assay (Thermo Scientific).
Discussion
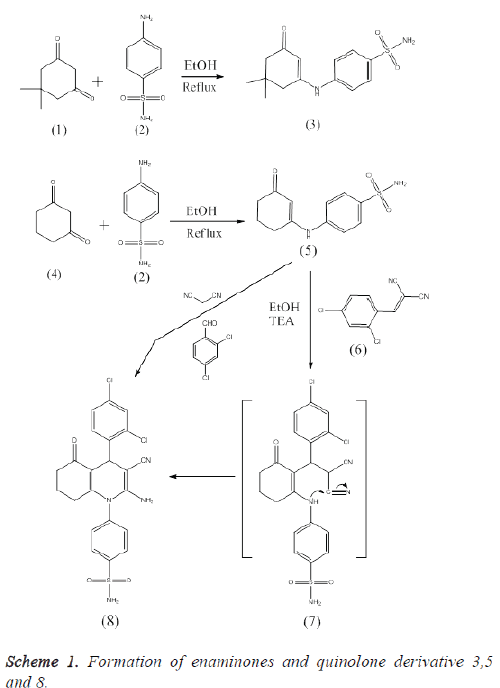
In this inquiry a string of enaminone results 3 and 5 and quinolone 7 attitude sulfonamide moiety and thiazoloquinazoline 13 were synthesized (Schemes 1 and 2) and biologically assessed for their in vitro cytoprotctive activity. Enaminone 3 was obtained by condensation of 5, 5- dimethyl- 1, 3-cyclohexandione 1 with sulfanilamide 2 [52].
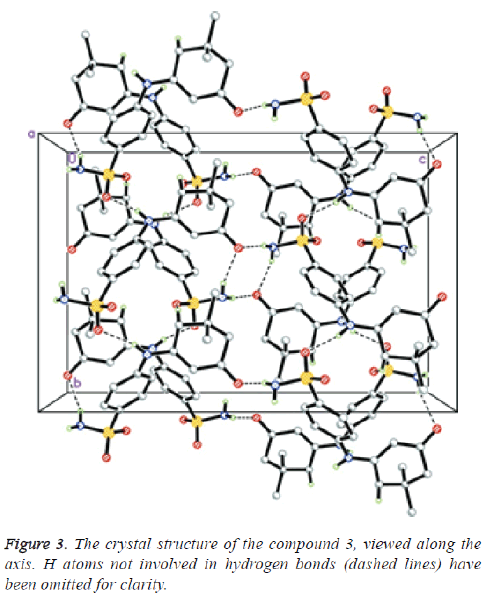
The assembly of compound 3 was verified by microanalysis and spectral data. Moreover compound 3 was recognized by Xray crystallographic analysis [56]. Alternatively, condensation of 1, 3-cyclohex-andione 4 with sulfanilamide 2 presented the corresponding enaminone 5, which upon reaction with 2-(2,4- dichlorobenzylidene) malononitrile 6, in ethanol containing a catalytic amount of triethylamine, bore 2-aminoquinoline-3- carbonitrile derivative 8 passing through the creation of the intermediate Michael type product 7, followed by intramolecular cyclization (Scheme 1).
Compound 8 was decidedly synthesized by alternative course linking one-pot condensation of the 2,4-dichlorobenzaldehyde, malononitrile, and enaminone 5 in a molar ratio (1:1:1) in refluxing ethanol comprising trimethylamine as catalyst. In this circumstance, formation of compound 8 exemplified in terms of early condensation of the aldehyde with malononitrile meet the expense of the triggered arylidenemalononitrile 6, followed by totaling of the enaminone 5 to the arylidenemalononitrile 6.
The consistent thiazoloquinoline 13 was gained in decent yield through reaction of 2-isothiocyanato derivative 9 with Lnorepheddrine (2-amino-1-phenylpropan-1-ol) 10 in dimethylformamide encompassing a catalytic quantity of trimethylamine. This reaction was ensue settled the creation of the intermediate 11 and 12 followed by intramolecular cyclization to bestow the thiazoloquinazoline derivative 13 (Scheme 2).
Biological activity
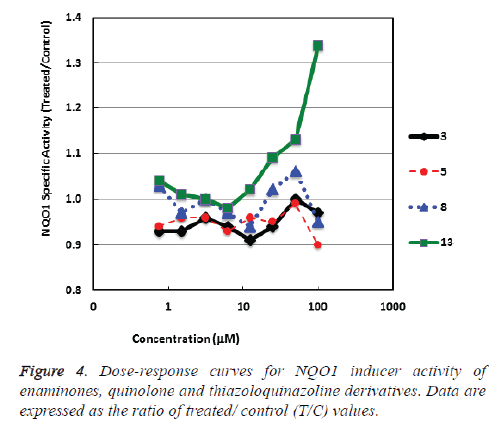
NAD(P)H: quinone acceptor oxidoreductase 1 (NQO1) is a cytoprotective enzyme which is triggered by electrophilic compounds via the Keap1/Nrf2 pathway [29]. Plentiful compounds which have been publicized to induce NQO1 have been successively found to the broadly cytoprotective and to meritoriously inhibit tumor formation in animal models [30,31]. We found that the quinazoline ring carrying a biologically active thiazole moiety (thiazoloquinazoline) 13 bare notable activity. While, the enaminone derivatives 3, 5 and quinolone 8 revealed weak NQO1 inducer activity (Table 1 and Figure 4).
| Compds No. | Induction Magnitude (Fold) |
|---|---|
| (4-(5, 5-Dimethyl-3-oxocyclohex-1-enyla-mino) benzenesulfonamide) (3). | 0.97 |
| (Oxocyclohexenylamino) benzenesulfonamide) (5). | 0.87 |
| (4-[2-Amino-3-cyano-4-(2,4-dichlorophenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]be-nzenesulfonamide) (8). | 0.95 |
| (1S, 2S)-3-methyl-2-phenyl-2, 3-dihydrothiazolo-[2, 3- b] quinazolin-5-one (13). | 1.34 |
Table 1: NQO1 inducer activity of enaminone derivatives 3, 5, quinoline derivative 8 and thiazoloquinazoline 13.
Conclusion
We testimony here the synthesis of exactly enaminone derivatives 3, 5, quinoline derivative 8 comprehending a biologically active sulfonamide moiety and thiazoloquinoline 13, it was evidently pragmatic that the fused three cyclic rings thiazoloquinazoline 13 is extra potent than enaminone derivatives 3, 5 and quinoline derivative 8. Still, joining the thiazolo moiety within a heterocyclic ring system (quinazoline) proliferations the inducer potency.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-VPP- 302.
The authors would like to express their gratitude and thanks to Albena T. Dinkova- Kostova and Maureen Higgins (Jacqui Wood Cancer Centre, Division of Cancer Research, Medical Research Institute, University of Dundee, Dundee UK) for doing the biological activity.
References
- Ahmedin J, Rebecca S, Elizabeth W, Taylor M, Jiaquan X, Michael JT. Cancer statistics. CA Cancer J Clin 2007; 57: 43-66.
- Srivastava A, Pandeya SN. “Indole” A versatile nucleus in pharmaceutical field. Int J Curr Pharm Rev Res 2011; 1: 1-17.
- Alsaid MS, Ghorab MM, Higgins M, Dinkova-Kostova AT,Shahat AA. NAD(P)H: Quinone Oxidoreductase 1 inducer activity of some enaminone derivatives. Biomed Res 2015; 26: 7-12.
- Patel NB, Agravat SN, Shaikh FM. Synthesis and antimicrobial activity of new pyridine derivatives-I. Med Chem Res 2011; 20: 1033-1041.
- Patel NB, Agravat SN. Synthesis and antimicrobial studies of new pyridine derivatives. Chem Heterocycl Comp 2009; 45: 1343-1353.
- Talaat IE, Shawkat AAM. Multi-component one-pot synthesis and antimicrobial activities of 3-methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydro-pyrazolo[5,4-b]pyrimidino[5,4-e]pyridine-5-one and Related Derivatives, Molecules 2012; 17: 14464-14483.
- Paronikyan EG, Noravyan AS, Dzhagatspanyan IA, Nazaryan IM, Paronikyan RG. Synthesis and anticonvulsant activity ofisothiazolo [5,4-b] pyrano (thiopyrano) [4,3-d]pyridine and isothiazolo [4,5-b]-2,7-naphthyridine Derivatives. Pharm Chem J 2002; 36: 465-467.
- Alice MRB, Alexandre RA, Luiz CSP,Júlio CB, Vinícius LC, Milene D M. Synthesis and antiviral activity of new 4-(phenylamino)/4-[(methylpyridin-2-yl)amino]-1-phenyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids derivatives, Med Chem Res 2007; 16: 352-369.
- Thomas JT, John TS, Robert MT, Theresa MW, Peter JF. Discovery of 3-{5-[(6-amino-1H-pyrazolo[3,4-b]pyridine-3-yl)methoxy]-2-chloroph-enoxy}-5-chlorobenzonitrile (MK-4965): A Potent, Orally Bioavailable HIV-1 non-nucleoside reverse transcriptase inhibitor with improved potency against key mutant viruses, J Med Chem 2008; 51: 6503-6511.
- Maria GM, Daniele Z, Valeria F, Luciano V, Maurizio F, Marco F. Antifungal and antimycobacterial activity of new N1-[1-aryl-2-(1H-imidazol-1-yl and 1H-1,2,4-triazol-1-yl)ethylidene]pyridine-2-carboxamidrazone derivatives: A combined experimental and computational approach. Arc Organic Chem 2004; 5: 231-250.
- Nasveld P, Kitchener S. Treatment of acute vivax malaria with tafenoquine. Trans R Soc Trop Med Hyg 2005; 99: 2-5.
- Leatham PA, Bird HA, Wright V, Seymour D, Gordon A. A double blind study of antrafenine, naproxen and placebo in osteoarthrosis. Eur J Rheumatol Inflamm 1983; 6: 209-211.
- Denny WA, Wilson WR, Ware DC, Atwell GJ, Milbank JB, Stevenson RJ. Anti-cancer 2,3-dihydro-1H-pyrrolo[3,2-f]quinoline complexes of cobalt and chromium, U.S. Patent 7064117 B2, 2006.
- Muruganantham N, Sivakumar R, Anbalagan N, Gunasekaran V, Leonard JT. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol Pharm Bull 2004; 27: 1683-1687.
- Maguire MP, Sheets KR, McVety K, Spada AP, Zilberstein A. A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J Med Chem 1994; 37: 2129-2137.
- Wilson WD, Zhao M, Patterson SE, Wydra RL, Janda L, Strekowski L, Schinazi RF. Design of RNA interactive anti-HIV agents: unfused aromatic intercalators. Med. Chem. Res. 1992; 2:102-110.
- Chakraborty B, Dutta D, Mukherjee S, Das S, Maiti NC. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur J Med Chem 2015; 102: 93-105.
- Lucjan S,Jerzy LM, Vidya AH, Agnieszka C,Marek TC. Synthesis andquantitative structure-activity relationship analysis of 2-(aryl or heteroaryl)quinolin-4-amines, a new class of anti-HIV-1 agents. J Med Chem 1991; 34: 1739-1746.
- Gopal M, Shenoy S, Doddamani LS. Antitumor activity of 4-amino and 8-methyl-4-(3diethylamino propylamino)pyrimido[4',5':4,5]thieno (2,3-b) quinolines. J Photochem Photobiol B 2003; 72: 69-78.
- Kim YH, Shin KJ, Lee TG, Kim E, Lee MS. G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem Pharmacol 2005; 69: 1333-1341.
- Zhao YL, Chen YL, Chang FS, Tzeng CC. Synthesis and cytotoxic evaluation of certain 4-anilino-2-phenylquinoline derivatives. Eur J Med Chem 2005; 40: 792-797.
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer 2002; 2: 188-200.
- Abdel-Gawad SM, El-Gaby MSA, Heiba HI, Aly HM, Ghorab MM. Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido[4,5-b]quinolinederivatives. J Chin Chem Soc 2005; 52: 1227-1236.
- El-Gaby MSA, Abdel-Gawad SM, Ghorab MM, Heiba HI,Aly HM. Synthesis and biological activity of some novel thieno[2,3-b]quinoline, quinolino[3',2':4,5] thieno[3,2-d]pyrimidine and pyrido[2',3':4,5] thieno[2,3-b]quinoline derivatives.PHOSPHORUS SULFUR 2006; 181: 279-297.
- GhorabMM, Abdel-HamideSG, FarragHA. Synthesis of novel quinolines, pyranoquinolines, furoquinolines, thienoquinoline and their effect on the ultrastructure of some pathogenic microorganisms, Acta Pol Pharm Drug Res 2001; 58: 175-184.
- Ghorab MM, Ragab FA, Noaman E, Heiba HI, Aboulmagd SA. Synthesis, anticancer and radioprotective activities of some new pyrazolo[3,4-d]pyrimidines containing amino acid moieties. Arzneimittelforschung 2009; 59: 96-103.
- Chen YL, Huang CJ, Huang ZY, Tseng CH, Chang FS. Synthesis and antiproliferative evaluation of certain 4-anilino-8-methoxy-2-phenylquinoline and 4-anilino-8-hydroxy-2-phenylquinoline derivatives. Bioorg Med Chem 2006; 14: 3098-3105.
- Ghorab MM,Ragab FA, Heiba HI,Ghorab WM. Design and synthesis of some novel quinoline derivatives as anticancer and radiosensitizing agents targeting VEGFR tyrosine kinase, J Hetero Chem 2011; 48: 1269-1279.
- Marganakop SB, Kamble RR, Hoskeri J, Prasad DJ,Meti GY. Facile synthesis of novel quinoline derivatives as anticancer agents, Med Chem Res 2014; 23: 2727- 2735.
- Li SY, Chen YL, Wang C, Tzeng CC. Synthesis and antiproliferative evaluation of certain pyrido[3,2-g]quinoline derivatives. Bioorg Med Chem 2006; 14: 7370-7376.
- Tseng CH, Chen YL, Lu PJ, Yang CN, Tzeng CC. Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Bioorg Med Chem 2008; 16: 3153-3162.
- Chen YL, Chen IL, Wang TC, Han CH, Tzeng CC. Synthesis and anticancer evaluation of certain 4-anilinofuro[2,3-b]quinoline and 4-anilinofuro[3,2-c]quinoline derivatives. Eur J Med Chem 2005; 40: 928-934.
- Ghorab MM, Noaman E, Ismail MM, Heiba HI, Ammar YA. Novel antitumor and radioprotective sulfonamides containing pyrrolo [2,3-d]pyrimidines. Arzneimittelforschung 2006; 56: 405-413.
- Ghorab MM, Ragab FA, Noaman E, Heiba HI, Galal M. Synthesis of certain new thiieno [2,3-d] pyrimidines as potential antitumor and radioprotective agents. Arzneimittelforschung 2006; 56: 553-560.
- Abou El Ella DA, Ghorab MM, Noaman E, Heiba HI, Khalil AI. Molecular modeling study and synthesis of novel pyrrolo[2,3-d]pyrimidines and pyrrolotriazolopyrimidines of expected antitumor and radioprotective activities. Bioorg Med Chem 2008; 16: 2391-2402.
- Ghorab MM, Osman AN, Noaman E, Heiba HI, Zaher NH. The utility of isothiocyanatothiophenes in the synthesis of thieno[2,3-d]pyrimidine derivatives as possible radioprotective and anticancer agents.PHOSPHORUS SULFUR 2006; 181: 1983-1996.
- Heiba HI, Ragab FA, Noaman E, Ghorab MM, Galal M. Synthesis of some novel sulfur containing triazolothienopyrimidines and biscompounds as possible antitumor and radioprotective agents. Arzneimittelforschung 2006; 56: 593-599.
- Ghorab MM. New biologically active N-(tetrahydrobenzothienopyrimidin-4-yl)-amino acids, thiourethane, sulfonamides and related compounds.PHOSPHORUS SULFUR2000; 165: 221-235.
- Ghorab MM, El-Sayed BS, Saker HM, Abd-Rabo MM. Synthesis and antitumor activity of some novel quinazoline derivatives bearing the biologically active thione moiety,Arzneimittel-Forschung 2007; 56: 665-670.
- Ghorab MM, Abdelhamide SG, Zeid MMA. Synthesis of some new thiadiazoleselena, triazine, thiazole and cyanopyridine derivatives with assay for their antitumor activity.PHOSPHORUS SULFUR1996; 112: 7-17.
- Ghorab MM, Abdel-Hamide SG, El-Gaby MSA. Synthesis and effect of some new [1,2,4]triazolo [4,3-a]quinazolin-5(4H)-ones and related compounds on Ehrlich Ascites Carcinoma cells. Acta Pharm 1999; 49: 1-10.
- GhorabMM, Ismail ZH, Abdel-GawadSM, Abdel AziemA. Antimicrobial activity of amino acid, imidazole, and sulfonamide derivatives of pyrazolo[3,4-d]pyrimidine. Heteroatom Chemistry 2004; 15: 57-62.
- Rostom SA, Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems.Bioorg Med Chem 2006; 14: 6475-6485.
- Supuran CT, Casini A, Mastrolorenzo A, Scozzafava A. COX-2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini Rev Medi Chem 2004; 4: 625-632.
- Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett 2004; 14: 217-223.
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002; 2: 55-75.
- Kivelä AJ, Kivelä J, Saarnio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol 2005; 11: 155-163.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008; 7: 168-181.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007; 15: 4336-4350.
- Bertucci A, Innocenti A, Zoccola D, Scozzafava A, Tambutté S. Carbonic anhydrase inhibitors. Inhibition studies of a coral secretory isoform by sulfonamides. Bioorg Med Chem 2009; 17: 5054-5058.
- Boyle NA, Chegwidden WR, Blackburn GM. A new synthesis of difluoromethanesulfonamides--a novel pharmacophore for carbonic anhydrase inhibition. Org Biomol Chem 2005; 3: 222-224.
- Ghorab MM, Ragab FA, Noaman E, Heiba HI, El-Hossary EM. Synthesis of some novel quinolones and pyrimido[ 4,5-b]quinolones bearing a sulfonamide moiety as potential anticancer and radioprotective agents. Arzneimittel- Forschung (Drug Res) 2007; 57: 795-803.
- Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM. Novel quinolones and pyrimido[4,5-b]quinolones bearing biologically active sulfonamide moiety as a new class of antitumor agents. EurJ Med Chem 2010; 45: 738-744.
- Ghorab MM, Alsaid MS, Abdel-Kader MS, Hemamalini M, Fun HK. Absolute configuration of (1S,2S)-3-methyl-2-phenyl-2,3-dihydrothiazolo[2,3- b]quinazolin-5-one. ActaCryst 2012; E68: o927-o928.
- Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem 1988; 169: 328- 336.
- Al-Said MS, Ghorab MM, Ghabbour HA, Quah CK, Fun HK. 4-[(5,5-Dimethyl-3-oxocyclo-hex-1-en-yl)amino]-benzene-sulfonamide. Acta Crystallogr Sect E Struct Rep Online 2012; 68: o2370.