ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 8
The adjunctive midazolam or magnesium sulfate to intrathecal bupivacaine analgesic effect in caesarean section: a randomized controlled trial
1Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran
2Student Research Committee, Fasa University of Medical Sciences, Fasa, Iran
3Shiraz University of Medical Sciences, Shiraz, Iran
- *Corresponding Author:
- Ali Hosseinipour
Noncommunicable Diseases Research Center
Fasa University of Medical Sciences, Iran
Accepted date: February 9, 2017
Background: Pain modulation is very important after operation, particularly for women who undergo caesarean section. Physiologic and psychological problems are the consequences of the improper pain management which raise the healthcare costs and prolong the recovery process.
Objectives: The aim of the present work is to assess the analgesic effect of combination of intrathecal midazolam or magnesium sulfate with bupivacaine in comparison to bupivacaine alone in parturient women who are candidate for non-emergent caesarean section.
Methods: Current study is a randomized controlled trial. 93 parturient women who were candidate for non-emergent caesarean section were selected. All individuals signed a written consent. By random block allocation they were divided to 3 groups. 10 mg bupivacaine 0.5% along with 2 mg midazolam intrathecally was administered for one group. An intrathecal injection of 10 mg bupivacaine 0.5% in addition to 100 mg magnesium sulfate was administered for second group. And finally the control group received an intrathecal injection of 10 mg bupivacaine 0.5% with 1 ml normal saline. The postoperative analgesic period, mean arterial pressure, heart rate, and frequency of nausea and vomiting were evaluated in 3 groups.
Results: The mean time from the anesthesia to start of pain sensation and demand for the first dose of analgesic was longer in midazolam and magnesium sulfate groups in comparison to the control group, although it wasn’t statistically significant. Furthermore, there was no significant difference between the pain-free parturient rates also the pain severity was not significantly different between the three groups 4 h after caesarean section. Hemodynamic changes such as mean arterial pressure and heart rate, the postoperative nausea and vomiting rate and the mean first and fifth minutes Apgar score did not differ in 3 groups.
Conclusion: The present work showed no benefit for intrathecal midazolam or magnesium sulfate combined with bupivacaine in women who undergo caesarean section.
Keywords
Caesarean section, Midazolam, Magnesium sulfate, Spinal anesthesia
Introduction
Pain modulation is very important after operation, particularly for women who undergo caesarean section. Physiologic and psychological problems are the consequences of the improper pain management which raise the healthcare costs and prolong the recovery process. Bupivacaine is the main agent used for spinal anesthesia in caesarean section. However, many researchers studied different classes of drugs as an adjuvant agent to reduce the side effects of bupivacaine and prolongation of analgesic period [1-4].
The opioid drugs are believed to have good analgesic effect when used as an adjuvant to intrathecal bupivacaine. Although, their important side effects such as pruritus, respiratory depression, urinary retention, nausea and vomiting have limited their use in practice [5-7]. Therefore, introducing nonopioid adjuvants for prolongation of the analgesic period is of particular interest in the field of anesthesiology.
Midazolam, a short-acting benzodiazepine, and magnesium sulfate, a non-competitive N-Methyl-D-Aspartate (NMDA) receptor antagonist, have been emerged as useful alternative agents for opioids in a number of studies [8-10]. Since a few number of studies have evaluated the analgesic effect of these agents in human [8-10]. The current study aimed to compare the analgesic effect of intrathecal midazolam or magnesium sulfate in combination with bupivacaine versus bupivacaine alone in parturient women undergoing non-emergent caesarean section.
Materials and Methods
Patient selection
Present work was a randomized controlled trial. The protocol of the study was approved by the ethical committee of Fasa University of Medical Sciences and was registered in the Iranian registry of clinical trials database (study code: IRCT2014102719145N2). 93 parturient women who were candidate for non-emergent caesarean section were selected. All individuals signed a written consent. The inclusion criteria were full-term pregnancy, non-emergent caesarean section (elective caesarean, previous caesarean, cephalopelvic disproportion, malpresentation) and the American Society of Anesthesiologists (ASA) physical status I or II. The exclusion criteria were contraindication for intrathecal injection, known allergy to midazolam or other benzodiazepines or systemic diseases (such as diabetes, hypertension and preeclampsia).
Study design
By random block allocation they were divided to 3 groups. 10 mg bupivacaine 0.5% along with 2 mg midazolam intrathecally was administered for one group (midazolam group). An intrathecal injection of 10 mg bupivacaine 0.5% in addition to 100 mg magnesium sulfate was administered for second group (magnesium group). And finally the control group received an intrathecal injection of 10 mg bupivacaine 0.5% with 1 ml normal saline.
Intervention
Before induction of spinal block, 15 mg/kg normal saline was infused for each parturient woman. After that, participants were placed in the sitting position and intrathecal injection was performed using an aseptic 23-gauge needle in the L3-L4 or L4-L5 intervertebral space. Parturients were turned into supine position right after intrathecal injection.
Outcome measurements
Postoperative analgesic period was defined as the time from the end of spinal block to the first request for analgesic agent by the parturient. The severity of pain was also investigated using 11-point Numerical Rating Scale (NRS-11) every 30 minutes for the next 4 h. Hemodynamic changes (mean arterial pressure and heart rate), frequency of nausea and vomiting were also compared between the study groups. A comparison of the first and fifth minutes Apgar score was made among the delivered infants of parturients in each group.
Statistical analysis
All statistical analyses were performed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). The quantitative data were presented as mean ± SD (median for non-parametric data). Baseline characteristics of participants in the study groups were compared using independent t-test (Mann-Whitney U test for non-parametric data). Chi-square test was used to compare the rate of pain-free parturient and postoperative nausea and vomiting between the study groups. Hemodynamic changes and the severity of pain were compared between the study groups using independent t-test or Mann-Whitney U test. Pvalue less than 0.05 was considered as statistically significant.
Results
The mean age of participants was 28.043 ± 5.285 years, which was not significantly different between the two groups (P=0.562). In addition, no significant differences between the two groups regarding baseline mean arterial pressure (P=0.730) and heart rate (P=0.406).
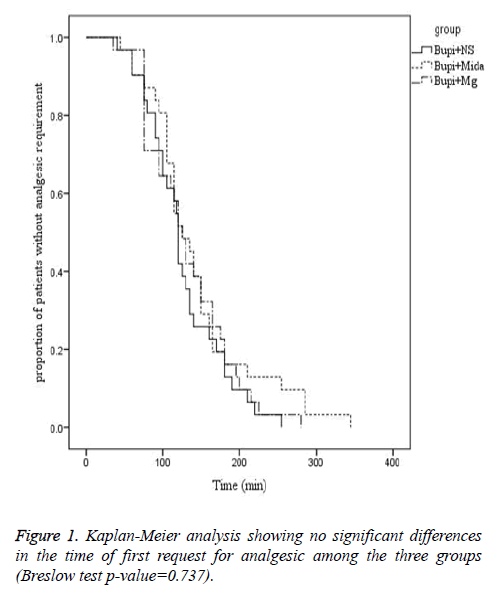
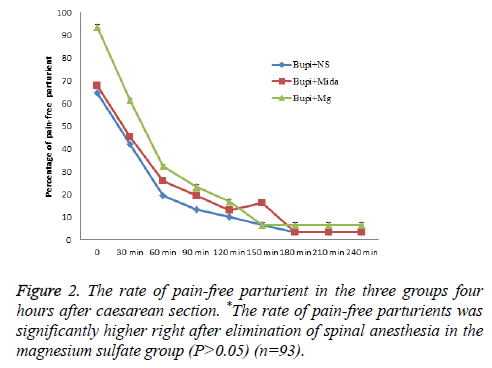
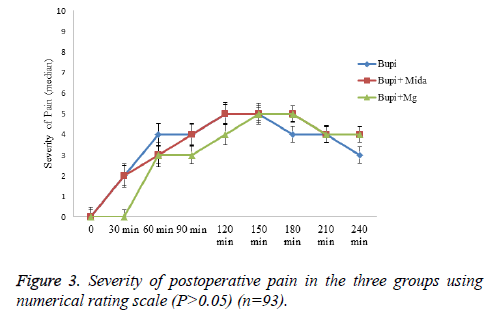
The mean time from the anesthesia to start of pain sensation and demand for the first dose of analgesic was longer in midazolam and magnesium sulfate groups in comparison to the control group, although it wasn’t statistically significant (144.03 ± 69.251, 132.10 ± 56.166 and 126.23 ± 50.10 minutes, respectively, P=0.737). Moreover, the rate of painfree parturients (P>0.05) and the severity of pain (P>0.05) were not significantly different between the three groups within four hours after caesarean section (Figures 1-3).
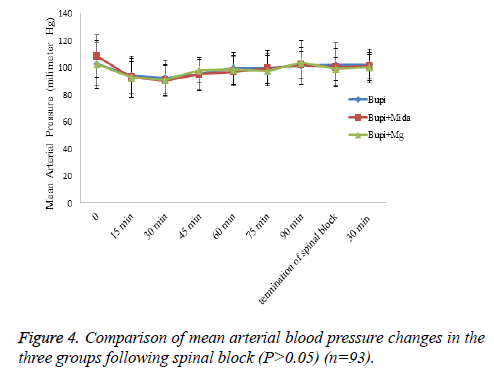
Hemodynamic variations such as mean arterial pressure and heart rate and frequency of postoperative nausea and vomiting were similar between the study groups (P>0.05) (Figures 4-6).
There was no significant difference regarding the mean first (0.439) and fifth (0.757) minuets Apgar score between the study groups (Table 1).
| Apgar score | Bupi | Bupi+Mida | Bupi+Mg | P-value |
|---|---|---|---|---|
| First minute | 9.097 ± 0.396 | 8.968 ± 0.482 | 8.968 ± 0.482 | 0.439 |
| Fifth minute | 9.129 ± 0.341 | 9.161 ± 0.374 | 9.097 ± 0.301 | 0.757 |
Table 1. Mean first and fifth minuets Apgar score in the three groups (n=93).
Discussion
In the current study, addition of intrathecal magnesium sulfate to bupivacaine showed significant relief of pain immediately after termination of spinal anesthesia. However, after 30 minutes there were no significant differences in the analgesic period and severity of pain in women received either adjuvant midazolam or magnesium sulfate or bupivacaine alone. Frequency of postoperative nausea and vomiting, hemodynamic changes and the first and fifth Apgar scores were also similar between the study groups.
Prevention of induction of central sensitization from peripheral nociceptive stimulation via blockade of NMDA channels is the proposed mechanism for analgesic effect of magnesium. To the authors’ knowledge, analgesic effect of intrathecal injection of magnesium sulfate and midazolam has not been compared with each other previously. However, a number of studies investigated the analgesic effect of intrathecal magnesium sulfate with opioids. Khezri et al. reported a significant delay in the onset of sensory and motor blockade by adding 0.5 ml magnesium 10% to 15 mg bupivacaine. The time to first analgesic requirement was shorter in patients receiving magnesium compared to fentanyl in that study [11]. A similar study by Malleeswaran et al. reported longer analgesic period and lower rate of analgesic requirements in parturients with mild pre-eclampsia who received 50 mg magnesium sulfate versus 25 mg fentanyl [12]. Enhanced analgesic effect with adjunctive magnesium sulfate rather than morphine to bupivacaine was also reported by Sun et al. [13]. Our study showed significant relief of pain with adjunctive magnesium sulfate immediately after termination of spinal anesthesia compared with adjunctive midazolam to bupivacaine.
The analgesic effect of intrathecal midazolam in women undergoing caesarean section has been investigated in only one randomized clinical trial previously. Karbasfrushan et al. [14] in a similar study reported that adding 2 mg/ml of midazolam to bupivacaine is associated with a significant relief of pain at 15 and 120 minutes after caesarean section. Of note, frequency of postoperative nausea and vomiting was higher than the control group in this study.
Two mechanisms have been suggested for analgesic effect of intrathecal midazolam; release of endogenous opioid acting at spinal delta receptors and reduction of excitatory GABAmediated neurotransmission [15,16]. The analgesic effect of adjunctive therapy with intrathecal midazolam has been investigated in patients undergoing different surgical procedures. Talebi et al. [17] showed that injection of 1 mg midazolam in combination with lidocaine is effective and safe for controlling postoperative pain in inguinal herniorrhaphy. Intrathecal midazolam in combination with sufentanil was also reported to be effective for enhancing analgesia in labor pain [18]. The analgesic effect of Midazolam as an adjunctive to bupivacaine has been investigated in two studies. In the Chattopadhyay’s study, it was shown that adjunctive therapy with midazolam significantly increased the duration of analgesia and decreased the incidence of postoperative nausea and vomiting in patients undergoing infraumbilical surgery [19]. Shadangi et al. reported similar findings without excessive rate of adverse effects [20].
Although adding intrathecal midazolam to a local anesthetic showed longer analgesia in previous studies, our study failed to illustrate any benefit from intrathecal midazolam combined with bupivacaine in women undergoing caesarean section. The differences in pain threshold and dose of drug can be a potential reason for our findings.
The Apgar score of new-borns was also similar between the three groups in our study, which indicates safety of intrathecal administration of midazolam and magnesium sulfate for newborns.
Our study had certain limitations including inability to test different doses of intrathecal midazolam and magnesium sulfate on duration of analgesia and postoperative complications and not including the number of gravidities of parturient women. Further studies with considering these points are needed to determine the effect of intrathecal midazolam and magnesium sulfate in women undergoing caesarean section.
Conclusion
The present work showed no benefit for intrathecal midazolam or magnesium sulfate combined with bupivacaine in women who undergo caesarean section.
References
- Tan PH, Chia YY, Lo Y, Liu K, Yang LC, Lee TH. Intrathecal bupivacaine with morphine or neostigmine for postoperative analgesia after total knee replacement surgery. Can J Anaesth 2001; 48: 551-556.
- Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology 1989; 71: 535-540.
- Khezri MB, Ghasemi J, Mohammadi N. Evaluation of the analgesic effect of ketamine as an additive to intrathecal bupivacaine in patients undergoing caesarean section. Acta Anaesthesiol Taiwan 2013; 51: 155-160.
- Faiz SH, Rahimzadeh P, Sakhaei M, Imani F, Derakhshan P. Anesthetic effects of adding intrathecal neostigmine or magnesium sulphate to bupivacaine in patients under lower extremities surgeries. J Res Med Sci 2012; 17: 918-922.
- Chun DH, Kil HK, Kim HJ, Park C, Chung KH. Intrathecal meperidine reduces intraoperative shivering during transurethral prostatectomy in elderly patients. Korean J Anesthesiol 2010; 59: 389-393.
- Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anaesthesia for Caesarean section. Br J Anaesth 2002; 88: 379-383.
- Duale C, Frey C, Bolandard F, Barriere A, Schoeffler P. Epidural versus intrathecal morphine for postoperative analgesia after caesarean section. Br J Anaesth 2003; 91: 690-694.
- Sen A, Rudra A, Sarkar SK, Biswas B. Intrathecal midazolam for postoperative pain relief in caesarean section delivery. J Indian Med Assoc 2001; 99: 683-684, 686.
- Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg 2004; 98: 1521-1527.
- Boussofara M, Carles M, Raucoules-Aime M, Sellam MR, Horn JL. Effects of intrathecal midazolam on postoperative analgesia when added to a bupivacaine-clonidine mixture. Reg Anesth Pain Med 2006; 31: 501-505.
- Khezri MB, Yaghobi S, Hajikhani M, Asefzadeh S. Comparison of postoperative analgesic effect of intrathecal magnesium and fentanyl added to bupivacaine in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Taiwan 2012; 50: 19-24.
- Malleeswaran S, Panda N, Mathew P, Bagga R. A randomised study of magnesium sulphate as an adjuvant to intrathecal bupivacaine in patients with mild preeclampsia undergoing caesarean section. Int J Obstet Anesth 2010; 19: 161-166.
- Sun J, Wu X, Xu X, Jin L, Han N, Zhou R. A comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int J Obstet Anesth 2012; 21: 310-316.
- Karbasfrushan A, Farhadi K, Amini-Saman J, Bazargan-Hejazi S, Ahmadi A. Effect of intrathecal midazolam in the severity of pain in cesarean section: a randomized controlled trail. Iran Red Crescent Med J 2012; 14: 276-282.
- Goodchild CS, Guo Z, Musgreave A, Gent JP. Antinociception by intrathecal midazolam involves endogenous neurotransmitters acting at spinal cord delta opioid receptors. Br J Anaesth 1996; 77: 758-763.
- Kohno T, Wakai A, Ataka T, Ikoma M, Yamakura T. Actions of midazolam on excitatory transmission in dorsal horn neurons of adult rat spinal cord. Anesthesiology 2006; 104: 338-343.
- Talebi H, Yazdi B, Alizadeh S, Moshiry E, Nourozi A, Eghtesadi-Araghi P. Effects of combination of intrathecal lidocaine and two doses of intrathecal midazolam on post-operative pain in patients undergoing herniorrhaphy: a randomized controlled trial. Pak J Biol Sci 2010; 13: 1156-1160.
- Salimi A, Nejad RA, Safari F, Mohajaerani SA, Naghade RJ. Reduction in labor pain by intrathecal midazolam as an adjunct to sufentanil. Korean J Anesthesiol 2014; 66: 204-209.
- Chattopadhyay A, Maitra S, Sen S, Bhattacharjee S, Layek A, Pal S. A study to compare the analgesic efficacy of intrathecal bupivacaine alone with intrathecal bupivacaine midazolam combination in patients undergoing elective infraumbilical surgery. Anesthesiol Res Pract 2013; 2013: 567134.
- Shadangi BK, Garg R, Pandey R, Das T. Effects of intrathecal midazolam in spinal anaesthesia: a prospective randomised case control study. Singapore Med J 2011; 52: 432-435.