Keywords |
| Bilirubin; Antibacterial effect; Newborn; Jaundice; Sepsis |
Introduction |
| Bilirubin is an end product of heme metabolism which is
released in association with the breakdown of hemoglobin.
Previously, the products of heme metabolism considered
negligible waste products. But unlike the other livings, only in
mammals biliverdin undergoes additional metabolism that
consumes the energy to produce bilirubin [1]. Elevated serum
bilirubin levels are associated with the manifestation named as
hyperbilirubinemi-jaundice in many newborn [2]. |
| The natural antioxidant characteristics of bilirubin were first
discovered by Stocker and colleagues [3]. Recent studies have
suggested that bilirubin causes oxidative damage at
pathological levels but exerts antioxidant effects at
physiological levels [4]. Bilirubin can trigger apoptosis of cell
cultures and stimulates the inflammatory response to
lipopolysaccharide [5]. Bilirubin protected mice subjected to
experimental sepsis [6]. Santangelo and colleagues found that
bilirubin exhibited antiviral activity when added to infected
cell cultures [7]. Bilirubin can be elevated in cases of sepsis,
intra-abdominal abscesses from urological, gynecological or
gastroenterological origins, and antiviral therapy [8]. |
| Bilirubin has been suggested to be an effective antibacterial,
but few data are available. Thus, we explored this topic by
measuring the antibacterial effects of bilirubin on certain
Gram-negative bacteria using both agar dilution and liquid
microdilution methods. |
Materials and Methods |
| Strains and bacterial identification |
| Gram-negative bacterial agents of sepsis were divided into four
test groups according to their species. Twenty-five strains each
of Escherichia coli, Klebsiella pneumoniae, Acinetobacter
baumannii, and Pseudomonas aeruginosa strains, isolated from
various clinical samples in the medical microbiology
laboratory of our hospital, were evaluated. Cultures were
inoculated on blood agar, eosin methylene blue (EMB) agar, or
chocolate agar, depending on the sample type and incubated by
standard conventional microbiological methods [9]. Species
identification was performed using the Vitek 2 automated
system (bioMérieux, Marcy-l'Étoile, France), and evaluated
based upon the recommendations of the Clinical Laboratory
Standards Institute (CLSI) [10]. Patient laboratory records were examined retrospectively. If several isolates were
obtained from the same patient, only one was included in the
evaluation. |
| Medium preparation and dilution studies |
| Agar dilution: Stock solutions of bilirubin (5 mg, 10 mg, and
15 mg/dl) (AppliChem, GmbH, Darmstadt, Germany)
containing EDTA and NaHCO3 were prepared and mixed in
the appropriate amounts with Mueller-Hinton agar (Oxoid,
Hampshire, England) at 50°C, which was used to prepare solid
culture plates with 4-mm-thick agar. Bacterial suspensions of
McFarland turbidity 0.5 were prepared, and 5-μl aliquots were
spotted onto the agar plates within circles 5 mm in diameter
(final bilirubin concentrations: 5, 10, and 15 mg/dl). Bacterial
growth was evaluated after 18 h of incubation at 35°C. Control
plates contained no bilirubin. |
| Microdilution Test: Bacterial suspensions of McFarland
turbidity 0.5 were prepared from 24-h cultures using the Densi-
Check 2 system (bioMerieux, Durham, NC, USA) and diluted
1/100 to 5 × 105 colony-forming units (cfu)/ml, as suggested
by CLSI [11]. Growth, negative, and positive controls were
included. Aliquots of bilirubin stock solutions were added to
120 μl liquid brain heart infusion medium in sterile microplates
using an automatic pipette. Serial dilution yielded final
bilirubin concentrations of 64, 32, 16, 8, 4, 2, 1, and 0.5 μg/ml.
Bacterial suspensions were added to 5 × 105 cfu/ml, and initial
optical densities (ODs) at 450 nm were recorded. After an 18-h
incubation at 35°C, OD changes were recorded using an EPOC
(Biotek, USA) device. The concentration of bilirubin that
reduced the final OD value to 50% that of the growth control
was defined as the MIC. |
| Statistical Analysis: E. coli ATCC 25922, K. pneumoniae
ATCC 13883, A. baumannii ATCC 19606, and P. aeruginosa
ATCC 27853 were used as control isolates. Agar and
microdilution tests was carried out in triplicate using three
samples for all isolates under the same conditions. Descriptive
statistics are presented as mean ± standard deviation (SD). One
Way ANOVA test was used for comparing the data. Statistical
significance was assumed if p<0.05. All statistical evaluation
was performed using SPSS (Version 17.0 for Windows; SPSS,
Inc., Chicago, IL, USA). |
Results |
| Liquid microdilution |
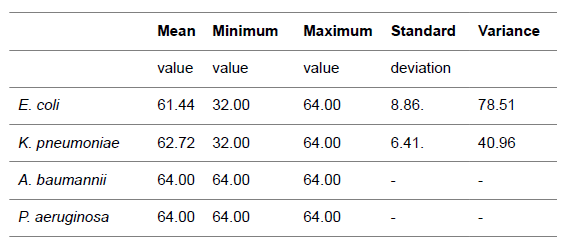
| The average bilirubin MICs were 61.44, 62.72, ≥ 64.00, and ≥
64.00 μg/mL for E. coli, K. pneumoniae, A. baumannii, and P.
aeruginosa respectively. Between E. coli and K. pneumoniae
MIC values was found differences but there was no statistical
significant (p>0.05). There was no difference between A.
baumannii, and P. aeruginosa (Table 1). The detected MIC
levels were found higher than the amount required that can be
used in antibiotherapy. |
 |
| Table 1: Statistical analysis of MIC values. |
| Agar dilution |
| The E. coli isolates, which had different resistance profiles, and
the control E. coli ATCC 25922 were resistant to bilirubin at all
levels tested. This was also true of the K. pneumoniae isolates
and ATCC 13883 control, the A. baumannii isolates and ATCC
19606 control, and the P. aeruginosa isolates and ATCC 27853
control. Moreover, there was no sensitive clinical isolate in the
study at all levels tested. |
Discussion |
| Despite extensive work, it remains unclear whether bilirubin, a
heme catabolite, is a non-functional end-product or a
biologically significant compound [4]. However, studies on the
antioxidant properties of bilirubin have defined treatment
threshold values [12]. Certain questions have arisen: “Are
phototherapy and blood changes over-prescribed?”, “To what
level should the plasma bilirubin level of a newborn be
decreased?”, and “Are we interfering with a useful defense
mechanism?” |
| Although advances in neonatal care have reduced
complications in and increased the survival of preterm infants,
neonatal sepsis is still associated with significant mortality and
morbidity, particularly, of low birth-weight infants (<1,500 g)
[13]. Such infants have high bilirubin levels. Maisels et al.
found that jaundice in 306 newborns was neither the cause of
nor a risk factor for bacteremia or sepsis [14]. However, a
Nigerian study found that septicemia in preterm infants was
associated with high bilirubin levels and high-level mortality
[15]. In Turkey, Ergür et al. reported that 11% of infants with
prolonged jaundice experienced sepsis and other infections
[16]. |
| Increased interest in the antioxidant effects of bilirubin has
encouraged work on the antibacterial properties of biliverdin/
bilirubin, which are heme catabolites. Overhaus et al. found
that intraperitoneal injection of biliverdin protected mice from
sepsis-induced inflammation and intestinal dysmotility [12].
Biliverdin decreased intestinal morbidity by selectively
decreasing inflammation. Wang et al. found that a single
intravenous injection of bilirubin protected mice from
mortality and liver dysfunction induced by injection of E. coli
endotoxin [6]. Recently, Lanone et al. showed that mouse
mortality caused by endotoxins decreased as the bilirubin level
increased [17]. Supporting such data [18], the presence of high
levels of oxidative metabolites of bilirubin in the urine of adult
patients with sepsis suggests that bilirubin degradation
parallels the oxidative stress of sepsis [19]. Goddard et al. inoculated group B streptococcus (GBS), coagulase-negative
staphylococcus (CNS), and E. coli (all isolated from blood
cultures) into media containing bilirubin at concentrations of
0-100 μmol/L [20]. E. coli was bilirubin-resistant, while GBS
and CNS were not. It was suggested that physiological
jaundice prevented the growth of Gram-positive bacteria such
as GBS and CNS, thus protecting against sepsis in premature
infants. In our study we evaluated the effects of bilirubin on
Gram-negative agents of sepsis using both agar and liquid
dilution methods. All tested bacteria grew at all bilirubin
concentrations used; thus, bilirubin had no antibacterial effect. |
| Santangelo et al. investigated the antiviral effect of bilirubin in
vivo conditions. Bilirubin at 1-10 μM concentrations
significantly decreased the growth of human herpes virus type
1 (HSV-1) and enterovirus 71 (EV 71) in Hep-2 and Vero cells
[7]. In our study the absence of any in vitro antibacterial effect
of bilirubin may indicate that bilirubin is effective only in vivo.
Furthermore, the antioxidant characteristic of bilirubin may
have an in vivo antibacterial effect. Also in vivo detection of
the any antibacterial effect of bilirubin may be useful for the
regulation of treatment protocols in neonatal jaundice. In
addition, we evaluated only low numbers of bacterial isolates.
Device constraints rendered us unable to test bilirubin
concentrations greater than 64 μg/ml (liquid culture) or 15
mg/dl (agar culture). Moreover, bilirubin at concentrations
exceeding 15 mg/dl is toxic to newborns. |
Conclusion |
| Bilirubin had no in vitro antibacterial effect on E. coli, K.
pneumoniae, A. baumannii, or P. aeruginosa. As explained
above, in vivo work using animal models is required. Also,
different microorganisms should be tested. |
References |
- Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdinreductase antioxidant cycle. Pediatrics 2004; 113: 1776-1782.
- Watchko JF, Maisels MJ. Neonatal jaundice. Semin Neonatal 2002; 7: 101-103.
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043-1046.
- Brito MA, Brites D, Butterfield DA. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res 2004; 1026: 33-43.
- Fernandes A, Falcao AS, Silva RF, Gordo AC, Gama MJ, et al. Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin. J Neurochem 2006; 96: 1667-1679.
- Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004; 40: 424-433.
- Santangelo R, Mancuso C, Marchetti S, Di Stasio E, Pani G, et al. Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro. Front Pharmacol 2012; 3: 36.
- Buyukasik Y, Akman U, Buyukasik NS, Goker H, Kilicarslan A, et al. Evidence for higher red blood cell mass in persons with unconjugated hyperbilirubinemia and Gilbert’s syndrome. Am J Med Sci 2008; 335: 115-119.
- Koneman E, Winn W, Allen S, Janda W, Procop G, et al. Koneman’sColor Atlas and Textbook of Diagnostic Microbiology. Sixth edition, Lippincott Williams & Wilkins, USA, 2006.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute. Wayne, PA, 2014.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Tenth Edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute. Wayne, PA, 2015.
- Overhaus M, Moore AB, Barbato J, Behrendt F,Doering J, et al. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. Am J PhysiolGastrointest Liver Physiol 2006; 290: G695-703.
- Kaufman D, Fairchild KD. Clinical Microbiology of Bacterial and Fungal Sepsis in Very-Low-Birth-Weight Infants. ClinMicrobiol Rev 2004; 17: 638-680.
- Maisels MJ, Kring E. Risk of sepsis in newborns with sever hyperbilirubinemia. J Pediatr 1992; 90: 741-743.
- Owa J, Dawodu AH. Neonatal jaundice among Nigerian preterm infants. West Afr J Med 1990; 9: 252-257.
- Ergür AT, Murat M, Leylek OA, Cevit Ö, İçağasıoğlu D, et al. Evaluation Of Neonates Diagnosed As Prolonged Hyperbilirubinemia In The Our Neonatology Unit. TurkiyeKlinikleri J Pediatr 1997; 6: 167-172.
- Lanone S, Bloc S, Foresti R, Almolki A, Taillé C, et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J 2005; 19: 1890-1892.
- Yamaguchi T, Horio F, Hashizume T, Tanaka M, Ikeda S, et al. Bilirubin is oxidized in rats treated with endotoxin and acts as a physiological antioxidant synergistically with ascorbic acid in vivo. BiochemBiophys Res Commun 1995; 214: 11-19.
- Otani K, Shimizu S, Chijiiwa K, Yamaguchi K, Kuroki S, et al. Increased urinary excretion of bilirubin oxidative metabolites in septic patients: a new marker for oxidative stress in vivo. J Surg Res 2001; 96: 44-49.
- Goddard M, Hansen R, Karcher AM, Munro MJ, Berry SH, et al. Bilirubin has anti-bacterial properties against gram-positive bacteria: A potential benefit of physiological jaundice? Gastroenterology 2011; 140: S-941.
|
