ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
The anti-cancer and anti-metastasis effects of phytochemical constituents from Leucaena leucocephala
Liang-Chien She1#, Chi-Ming Liu2#, Ching-Tong Chen3, Hsing-Tan Li4, Wei-Jen Li5* and Chung-Yi Chen4*
1Department of Rehabilitation Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
2Department of Nursing, Tzu Hui Institute of Technology, Pingtung County, Taiwan
3Department of Health and Beauty, Shu-Zen Junior College of Medicine and Management, Kaohsiung, Taiwan
4School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan
5Department of Nursing, Fooyin University, Kaohsiung, Taiwan
#These authors contributed equally
- *Corresponding Authors:
- Chung-Yi Chen
Department of Nutrition and Health Science
School of Medical and Health Sciences
Fooyin University, Taiwan - Wei-Jen Li
Department of Nursing
Fooyin University, Taiwan
Accepted date: November 25, 2016
The objective of this study was to evaluate the anti-cancer effects of compounds obtained from the plant Leucaena leucocephala in human chronic myelogenous leukaemia, prostate, liver, tongue, lung, melanoma, gastric and colon adenocarcinoma cancer cell lines. The data from the chemical analyses has identified 14 compounds belonging to prenols, sterol, flavonoids, coumarins and triterpene derivatives. The IC50 values of pheophorbide a methyl ester, pyropheophorbide and pheophytin-a are 2.6, 3.69 and 1.89 μM in AGS cell line. Pheophorbide a methyl ester, pyropheophorbide and pheophytin-a also displayed anti-migration and anti-invasion activity in AGS cell line. These results indicated that investigation on the anti-proliferation, anti-migration and anti-invasion effects of components from Leucaena leucocephala.
Keywords
Leucaena leucocephala, Anti-cancer, Mimosaceae
Introduction
Cancer is the leading cause of death in the developed and developing countries. The potential anti-cancer agent from herb is an effective approach for the development of new drugs for treatment cancer patients [1-3]. Leucaena leucocephala is mimosoid tree also called white lead tree native to southern Mexico and northern Central America belonged to Mimosaceae family. The leaves and seeds of Leucaena leucocephala contain lipids, crude protein, tannin, carbohydrates, and mimosine [4]. Mimosine is a non-protein amino acid and the chemical structure is similar to tyrosine. Mimosine is toxic to animals but mimosine can be removed by soaking the leaves in water for 24 h [5]. However, mimosine has anticancer activity in in human lung cancer cells by blocking cell cycle progression [6]. Previous study has shown that the extracts of the Leucaena leucocephala has anthelmintic, anti-oxidation and anti-diabetic activities [7-11]. The phytochemical data of Leucaena leucocephala revealed the presence of terpenes, flavonoids, coumarins and sterols [12]. In general, these components have many pharmacological activities in many studies.
The present work aims at isolating, screening and studying of these compounds from the leaves, seeds, green pods and brown pods of Leucaena leucocephala to evaluate their anti-cancer and pharmacological activity in different cancer cell lines.
Materials and Methods
Collection and identification of plant materials
The specimen of L. leucocephala was collected from Pingtung County, Taiwan in December, 2008. A voucher specimen was characterized by Dr. Jin-Cherng Huang of Department of Forest Products Science and Furniture Engineering, National Chiayi University, Chiayi, Taiwan and deposited in the School of Medical and Health Sciences, Fooyin University, Kaohsiung County, Taiwan.
Extraction and fractionation
The air-dried seeds of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room temperature and the MeOH extract (162.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel (800 g, 70~230 mesh) using n-hexane/acetone as eluent to produce 5 fractions. Part of fraction 1 (6.11 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (40:1), enriched with acetone to furnish 10 fractions (1-1~1-10). Fraction 1-1 (1.72 g) was re-subjected to Si gel chromatography, eluting with n-hexane/acetone (80:1) to obtain linoleic acid (58 mg). Part of fraction 3 (6.94 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (50:1) to obtain β-sitosterol (15 mg). The air-dried brown pods of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room temperature and the MeOH extract (171.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/acetone as eluent to produce 6 fractions. Part of fraction 1 (7.02 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (40:1) to obtain β-sitostenone (6 mg). Part of fraction 2 (8.49 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (10:1), then enriched with acetone to furnish 8 fractions (2-1~2-8). Fraction 2-3 (2.33 g) was re-subjected to Si gel chromatography, eluting with n-hexane/acetone (40:1) to obtain lupeol (74 mg). Part of fraction 6 (13.89 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (4:1), then enriched with acetone to obtain 5 α, 8 α- epidioxy-( 24 ξ)-ergosta-6, 22-dien-3 β-ol (43 mg). The airdried green pods of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room temperature and the MeOH extract (132.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/acetone as eluent to produce 10 fractions. Part of fraction 2 (2.67 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (50:1) to obtain ficaprenol-11 (21 mg). Part of fraction 3 (6.77 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (8:1), then enriched with acetone to furnish 5 fractions (3-1~3-5). Fraction 3-3 (1.33 g) was further purified by another silica gel column using n-hexane/acetone to obtain squalene (5 mg). Part of fraction 5 (7.42 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1) to obtain pheophorbide a methyl ester (12 mg). Part of fraction 6 (5.31 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1) to obtain pyropheophorbide-a (16 mg). Part of fraction 8 (4.91 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (5:1) to obtain trans-coumaric acid and cis-coumaric acid mixture (8 mg). The air-dried leaves of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room temperature and the MeOH extract (159.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/acetone as eluent to produce 7 fractions. Part of fraction 1 (6.62 g) was subjected to Si gel chromatography by eluting with n-hexane/ acetone (50:1) to obtain pheophytin-a (6 mg). Part of fraction 3 (7.56 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1), then enriched with acetone to furnish 12 fractions (3-1~3-12). Fraction 3-7 (2.97 g) was resubjected to Si gel chromatography, eluting with n-hexane/ acetone (40:1) to obtain 132-hydroxy-(132-S)-pheophytin-a (12 mg) and methyl-132-hydroxy-(132-S)-pheophorbide-b (9 mg). Part of fraction 6 (17.15 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (4:1) to obtain aristophyll-C (4 mg).
Cell lines and cell culture
Eight human cancer cell lines were used in these studies. All the cell lines were obtained from Bioresource Collection and Research Center (BCRC, Taiwan). DU145 is human prostate cancer cell line and incubated in Eagle's Minimum Essential Medium. Hep G2 is human liver hepatocellular carcinoma and this cell line was incubated in Eagle’s Minimum Essential Medium. SCC9 is oral squamous cell carcinoma and this cell line was incubated a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham’s F12 medium. H441 is human adenocarcinoma of the lung and incubated in RPMI-1640 medium. AGS is human gastric cancer cell line, and the cell was incubated in F-12K Medium. WiDr is colorectal adenocarcinoma and incubated in Eagle’s Minimum Essential Medium. K562 is human leukaemia cell line and incubated in Iscove's Modified Dulbecco's Medium. All cell lines were cultured in medium supplemented with 10% FBS (Gibco BRL) and antibiotic. These cell lines were routinely maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
XTT assay
Cells (1.0 × 104) were incubated with different concentrations of compounds in 200 μl of medium in 96-well plate for 24 hr. Cell viability was determined by the XTT assay. The yellow tetrazolium salt XTT is reduced to a formazan dye by dehydrogenase enzymes in active cells. The formazan produced is proportional to active cells. In brief, a freshly prepared XTT-PMS labelling mixture (50 μl) was added to the medium. The absorbance was measured at 450 nm.
Wound scratch migration assay
Cell migration assay was performed using a scratch woundhealing format. The anti-migration of test compounds was further examined in AGS cell line. When the cell is confluent in a 6-well plate, cells were then scratched and treated with different concentrations of compounds. Cell migration was monitored by microscopy.
Matrigel invasion chamber assay
The anti-invasion of test compounds was further examined in AGS cell line. Briefly, the upper sides of the transwell inserts (8 μm pore size) were coated with Matrigel per insert in 24- well plates. 5 × 105 cells were seeded in 500 μL serum-free medium in each upper chamber. The bottom well were added with different concentrations of compounds in 500 μL medium with 10% FBS. After incubation, the non-invasive cells were removed from the upper surface of the membrane with cotton swab, and the invasive cells on the under surface of the membrane. The under surface of the membrane were stained with Giemsa stain solution.
Statistical analysis
All experiments were carried out at three times and at least triplicate. The results were expressed as the average of the mean values ± Standard Deviation (SD). Analysis of the data was done with SigmaPlot software (Version 8.0, SPSS Scientific, Chicago, IL, USA) and SigmaStat (Version 2.03, SPSS Scientific) run on an IBM-compatible computer.
Results
Anti-proliferation of compounds in cancer cell lines
In the present study, we isolated 15 compounds from the different parts of Leucaena leucocephala. Linoleic acid and β- sitosterol were isolated from seeds. Lupeol, β-sitostenone and 5 α, 8 α-epidioxy-(24 ξ)-ergosta-6, 22- dien-3 β-ol from the brown pods. Pheophorbide a methyl ester, ficaprenol-11, squalene, mixture of cis- and trans-coumaric acid and pyropheophorbide-a were isolated from the green pods. Pheophytin-a, aistopyll-C, 132-hydroxy-(132-S)-pheophytin-a and methyl-132- hydroxy-(132-S)-pheophorbide b were isolated from the leaves. All compounds were tested in human chronic myelogenous leukaemia, prostate, liver, tongue, lung, melanoma, gastric and colon adenocarcinoma cancer cell lines. All compounds had no significant anticancer activity in human colon adenocarcinoma cell line (WiDr). Methyl-132-hydroxy- (132-S)-pheophorbide b and pyropheophorbide displayed board anti-cancer activity in many cancer cell lines including Hep G2 (liver), A375 (melanoma), DU145 (prostate), SCC9 (tongue), H44 (lung) and AGS (stomach). Moreover, pheophorbide a methyl ester, pyropheophorbide and pheophytin-a had significant anti-proliferation activity in AGS cell line. The IC50 values of compounds in different cancer cell lines were shown in Table 1.
| IC50 (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| DU145 | Hep G2 | SCC9 | H441 | A375 | AGS | WiDr | K562 | |
| Linoleic acid | 292.7 ± 10.32 | >500.0 | 19.2 ± 1.40 | 2.4 ± 0.40 | >500.0 | 92.1 ± 9.11 | >500.0 | 16.3 ± 1.45 |
| Pheophytin-a | 6.6 ± 0.75 | >500.0 | 5.8 ± 0.31 | >500.0 | 35.3 ± 6.40 | 3.69 ± 0.42 | >500.0 | 64.2 ± 5.30 |
| Pheophorbide a methyl ester | 81.2 ± 4.81 | >500.0 | 6.4 ± 0.57 | 0.5 ± 0.02 | >500.0 | 2.6 ± 0.43 | >500.0 | 2.9 ± 0.32 |
| 132-Hydroxy-(132-S)-pheophytin-a | 15.9 ± 1.17 | >500.0 | 46.4 ± 5.30 | >500.0 | >500.0 | 140.5 ± 6.4 | >500.0 | 0.7 ± 0.11 |
| Aristophyll-C | 29.3 ± 2.42 | >500.0 | 1 ± 0.01 | >500.0 | >500.0 | >500.0 | 440.8 ± 2.4 | 3.8 ± 0.78 |
| Methyl-132-hydroxy-(132-S)-pheophorbide b | 8.1 ± 1.00 | 157.8 ± 10.73 | 8.4 ± 0.78 | 68.4 ± 1.78 | 42.9 ± 5.40 | 9 ± 1.43 | >500.0 | 36.6 ± 4.43 |
| 5 α,8 α-epidioxy-(24 ξ)-ergosta-6, 22-dien-3 β-ol | 191.5 ± 7.78 | >500.0 | 13.5 ± 1.10 | NDx | >500.0 | 8.1 ± 1.1 | >500.0 | 2.8 ± 0.25 |
| β-sitosterol | 218.5 ± 10.40 | >500.0 | 14.2 ± 1.00 | 49.9 ± 4.32 | >500.0 | 56.9 ± 7.22 | >500.0 | 58.1 ± 5.32 |
| Lupeol | 126.3 ± 5.32 | >500.0 | 143 ± 8.38 | 63.4 ± 7.41 | >500.0 | 44.2 ± 4.40 | >500.0 | 19.2 ± 3.11 |
| β-sitostenone | >500.0 | >500.0 | >500.0 | 0.018 ± 0.00 | >500.0 | >500.0 | >500.0 | >500.0 |
| ficaprenol-11 | >500.0 | >500.0 | 436.2 ± 12.23 | 214.6 ± 9.34 | >500.0 | >500.0 | >500.0 | 22.0 ± 2.4 |
| Squalene | >500 | >500.0 | >500.0 | >500.0 | >500.0 | >500.0 | >500.0 | 14.1 ± 3.33 |
| cis- and trans-coumaric acid | >500 | >500.0 | 55.6 ± 7.41 | 392.1 ± 10.42 | >500.0 | >500.0 | >500.0 | 199.0 ± 9.42 |
| Pyropheophorbide-a | 24.5 ± 3.23 | 327.7 ± 13.33 | 0.716 ± 0.10 | 2.3 ± 0.41 | 29.5 ± 3.41 | 1.89 ± 0.22 | >500.0 | 0.5 ± 0.09 |
| Cisplatin | 1.8 ± 0.04 | NDx | NDx | 38.8 ± 2.78 | 96.5 ± 5.47 | 2.7 ± 0.11 | >500.0 | 0.2 ± 0.02 |
Table 1: Cytotoxicity of compounds obtained from Leucaena leucocephala in different cancer cell lines.
Pheophorbide a methyl ester, pyropheophorbide-a and pheophytin-a inhibit DNA synthesis, migration and invasion in AGS cell line
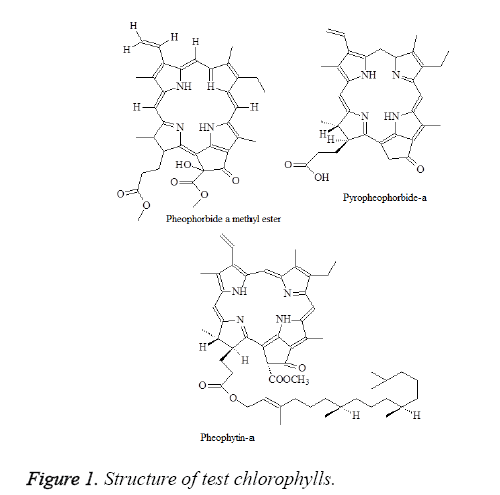
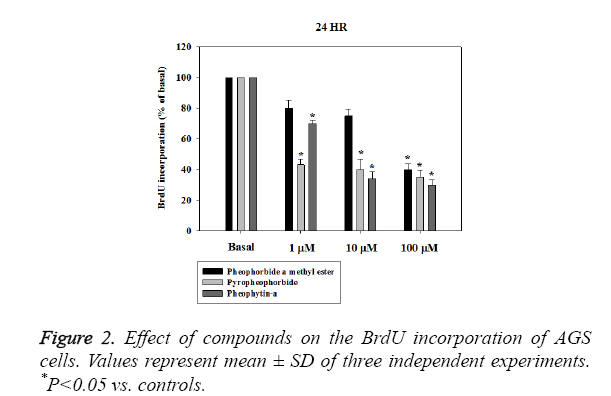
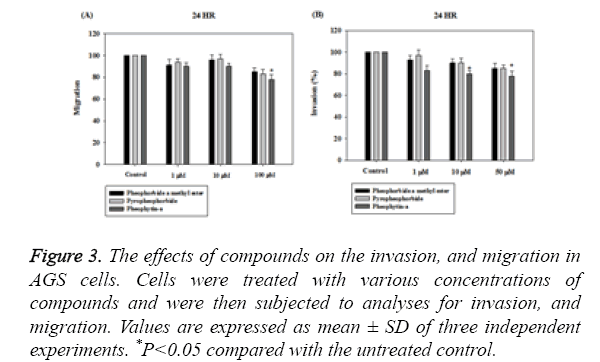
The chemical structure of pheophorbide a methyl ester, pyropheophorbide-a and pheophytin-a were shown in Figure 1.We next performed a BrdU incorporation assay to evaluate pheophorbide a methyl ester, pyropheophorbide and pheophytin-a DNA synthesis in AGS cell line. Pheophorbide a methyl ester, pyropheophorbide and pheophytin-a treatment for 24 h significantly decreased DNA synthesis in AGS cell line (Figure 2). We further examined the activity of anti-migration and anti-invasion in AGS cell line. The results indicated that the treatment of pheophorbide a methyl ester, pyropheophorbide-a and pheophytin-a for 24 h slightly inhibited migration and invasion (Figures 3A and 3B).
Figure 3: The effects of compounds on the invasion, and migration in AGS cells. Cells were treated with various concentrations of compounds and were then subjected to analyses for invasion, and migration. Values are expressed as mean ± SD of three independent experiments. *P<0.05 compared with the untreated control.
Discussion
Plants have many pure compounds with biological activities. A research has indicated that Leucaena leucocephala possess flavonoids with anti-oxidation activity [12]. In the present study, we revealed the isolation and identification of 14 pure compounds from the different parts of Leucaena leucocephala. These compounds were also evaluated the anti-cancer activity. We further examined the activity of anti-migration and antiinvasion of pheophorbide a methyl ester, pyropheophorbide a and pheophytin-a in AGS cell line.
Photodynamic therapy is based on photosensitizers. Photosensitizers are light-sensitive molecules. Photoactivation produces peroxidative reactions and make cell damage and death [13,14]. Pheophorbide a methyl ester, pyropheophorbide a and pheophytin-a were previously isolated from Phaeanthus ophthalmicus, Piper penangense and Zanthoxylum ailanthoides Sieb. and Zucc [15-17]. These compounds are photosensitizers with anti-cancer activity. In the present study, we did not evaluate the phototoxicity of the compounds in AGS cell line.
Gastric cancer is the second leading cause of cancer mortality in the world [18]. Lymphatic metastasis is commonly observed in gastric cancer. Many factors are involved in gastric cancer progression, such as Epidermal Growth Factor Receptor (EGFR) and PI3K/Akt/mTOR pathway [19-22]. Metastasis is a complex progress and actives by many steps such as decreased adhesion, increased motility and invasion and so on. Migration is also key step across the metastatic cascade. In the present study, we have shown that pheophorbide a methyl ester, pyropheophorbide a and pheophytin-a not only inhibit cancer cell proliferation but also decrease invasion and migration in gastric cancer cell line. The detail in vivo mechanism of pharmacological activities can be examined in the future.
Conclusion
The pure compounds were purified from the leaves, seeds, green and brown pods of Leucaena leucocephala. These compounds displayed important pharmacological activities such as anti-proliferation, anti-migration and anti-invasion in cancer cell lines. The information could provide a supportive role in the pharmaceutical field towards the development of new drugs.
Acknowledgements
This work was financially supported by Fooyin University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang H, Yin Y, Wang P, Xiong C. Current situation and future usage of anticancer drug databases. Apoptosis 2016; 21: 778-794.
- Dong X, Fu J, Yin X, Cao S, Li X. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother Res 2016; 30: 1207-1218.
- Wen S, Chen Y, Lu Y, Wang Y, Ding L. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia 2016; 112: 74-84.
- Sobale BN, Kharat ST, Prasad VL, Joshi AL, Rangnekar DV. Nutritive value of Leucaena leucocephala for growing bull calves. Trop Anim Health Prod 1978; 10: 237-241.
- Chou CH, Kuo YL. Allelopathic research of subtropical vegetation in Taiwan : III. Allelopathic exclusion of understory byLeucaena leucocephala (Lam.) de Wit. J Chem Ecol 1986; 12: 1431-1448.
- Chang HC, Lee TH, Chuang LY, Yen MH, Hung WC. Inhibitory effect of mimosine on proliferation of human lung cancer cells is mediated by multiple mechanisms. Cancer Lett 1999; 145: 1-8.
- Ademola IO, Idowu SO. Anthelmintic activity of Leucaena leucocephala seed extract on Haemonchus contortus-infective larvae. Vet Rec 2006; 158: 485-486.
- Singhal PC, Gupta RK, Singh JB, Joshi LD. Preliminary studies on hypoglycaemic and hypocholesterolemic activities of Leucaena leucocephala (Lam) de Wit. Indian J Med Res 1982; 76: 119-123.
- Benjakul S, Kittiphattanabawon P, Sumpavapol P, Maqsood S. Antioxidant activities of lead (Leucaena leucocephala) seed as affected by extraction solvent, prior dechlorophyllisation and drying methods. J Food Sci Technol 2014; 51: 3026-3037.
- von Son-de Fernex E, Alonso-Diaz MA, Mendoza-de Gives P, Valles-de la Mora B, Gonzalez-Cortazar M, Zamilpa A, Castillo Gallegos E. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Vet Parasitol 2015; 214: 89-95.
- Soares AM, de Araujo SA, Lopes SG, Costa Junior LM. Anthelmintic activity of Leucaena leucocephala protein extracts on Haemonchus contortus. Rev Bras Parasitol Vet 2015; 24: 396-401.
- Hassan RA, Tawfik WA, Abou-Setta LM. The flavonoid constitunts of Leucaena leucocephala. Growing in Egypt, and their biological activity. Afr J Trad Compl Alt Med Afr Netw Ethnomed 2014, 11: 67-72.
- Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J 2016; 473: 347-364.
- Ozog DM, Rkein AM, Fabi SG, Gold MH, Goldman MP. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol Surg 2016; 42: 804-827.
- Chou ST, Chan HH, Peng HY, Liou M J, Wu TS. Isolation of substances with antiproliferative and apoptosis-inducing activities against leukaemia cells from the leaves of Zanthoxylum ailanthoides Sieb.and Zucc. Phytomed 2011; 18: 344-348.
- Kamarulzaman FA, Shaari K, Ho AS, Lajis NH, Teo SH. Derivatives of pheophorbide-a and pheophorbide-b from photocytotoxic Piper penangense extract. Chem Biodivers 2011; 8: 494-502.
- Tan PJ, Ong CY, Danial A, Yusof HM, Neoh BK. Cyclic Tetrapyrrolic Photosensitisers from the leaves of Phaeanthus ophthalmicus. Chem Cent J 2011; 5: 32.
- Goral V. Etiopathogenesis of Gastric Cancer. Asian Pac J Cancer Prev 2016; 17: 2745-2750.
- Gong L, Debruyne PR, Witek M, Nielsen K, Snook A, Lin JE, Bombonati A, Palazzo J, Schulz S, Waldman SA. Bile acids initiate lineage-addicted gastroesophageal tumorigenesis by suppressing the EGF receptor-AKT axis. Clin Transl Sci 2009; 2: 286-293.
- Khanna P, Chua PJ, Bay BH, Baeg GH. The JAK/STAT signaling cascade in gastric carcinoma (Review). Int J Oncol 2015; 47: 1617-1626.
- Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact 2007; 165: 59-75.
- Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, Kanai F, Kawabe T, Omata M. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog 2009; 48: 703-712.