ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
The Application of DTI to Investigate Abnormalities in Schizophrenia and Alzheimer Disease
Martha L. Mora.1*, Carlos. A. Hurtado1, Vesna Prchkovska2, Paulo Rodrigues2, Juan José López Ibor3, Juan Arrazola3
1Cooperativa de Colombia University/School of Engineering and School Psychology Neuro-Spin Laboratory, Cali - Colombia
2 Mint Labs / Medical Innovation and Technology laboratories, Barcelona, Spain.
3Complutense University – Department Diagnostic for image – San Carlos Clinic Hospital, Madrid, Spain.
- *Corresponding Author:
- Martha L. Mora
Cooperativa de Colombia University
School of Engineering and School Psychology
Neuro-Spin Laboratory, Cali – Colombia
Spain.
Accepted date: June 07 2015
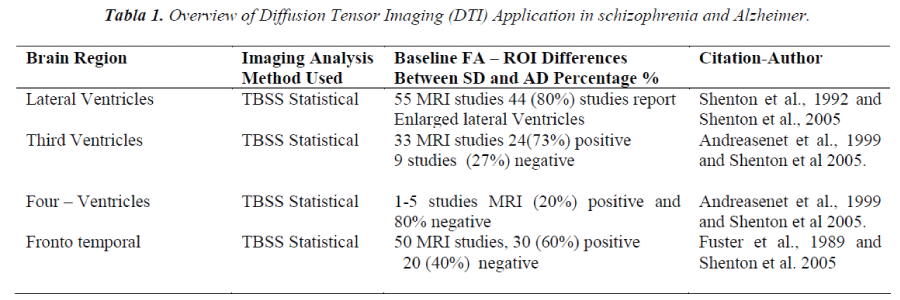
The objective of this study was to evaluate in individuals applications at clinical high risk of mental disorder with Diffusion Tensor Imaging (DTI-MRI). This provides an important opportunity for pathological mechanisms of Schizophrenia Disease (SD) and Alzheimer Disease (AD), related to mental disorder. All disease is associated with abnormal activity distributed spatially in neural systems with mediate the motor and cognitive manifestations of this disorder in combination with processing and visualization techniques for Diffusion Tensor Magnetic Resonance Imaging (DTI-MRI). It is possible that metabolic MRI studies have demonstrated that this illness is characterized by a set of reproducible functional brain networks that correlate with these clinical features. The time at which these abnormalities appear is unknown, as is their relationship to concurrent clinical and indices of disease progression. We therefore need to set vivo biomarkers that can distinguish the differing pathologies and their contribution to the clinical features of the conditions. Diffusion tensor imaging (DTI) utilizes the anisotropic nature of diffusion in neuronal white matter tracts. With neuronal degeneration, the mean diffusivity (MD) increases with the loss of structural barriers that restrict normal diffusion, and diffusion becomes less directionally oriented, which is associated with a reduction in fractional anisotropy (FA). Areas of reduced FA in subjects with lateral ventricle were found primarily in occipital parietal area with white matter tracts; in AD, it was also associated with reduced FA in the points the left thalamus.
Keywords
Mental disorder, Diffusion Tensor Imaging, Schizophrenia disease, Alzheimer disease
Introduction
In recent years, there has been an increasingly high clinical risk of mental disorder with DTI-MRI in clinical applications with Schizophrenia Disease (SD) [1] and Alzheimer Disease (AD).
We need to set vivo biomarkers that can distinguish the differing pathologies and their contribution to the clinical features of the conditions. These pathologies was rekindled in the 1970s, when the first computer-assisted tomography (CT) study showed enlarged lateral ventricles in schizophrenia and a prominent feature of early Alzheimer’s Disease (AD) [2] attributed to degeneration of medial temporal and parietal brain regions, including the retrosplenial cortex (RSC). With the introduction of magnetic resonance imaging (MRI) studies, the first MRI study of schizophrenia was conducted in 1984[3].
Since that time, there have been many improvements in MR acquisition and image processing, such as: introduction of Positron Emission Tomography (PET), Functional MRI (fMRI) and Diffusion Tensor Imaging (DTI). These advances have an appreciation of the critical role that the brain has in abnormalities of mental disorder-related diseases.
Previous studies investigating DTI changes in Schizophrenia Disease (SD) and Alzheimer Disease (AD) used conventional region of interest (ROI) or voxel-based on DTI-MRI [3]. Some studies found widespread FA changes in comparison with healthy controls. The most consistent finding has been that of reduced FA in the region of the inferior longitudinal fasciculus (ILF) in Schizophrenia (SD) also associated with reduced FA in the points and left thalamus, in comparison with AD.
To investigate patterns in clinical biomarkers that can distinguish the differing pathologies and their contribution to the clinical features high risk of mental disorder, different levels of investigation clinical can be distinguished: first, a patient with premature dementia in present studies, (SD discussed below), has experienced lateral ventricle [4] enlargement, medial temporal lobe volume reduction (including amygdala-hippocampal complex gyrus) and specific white matter with volume reductions that are especially prominent in the superior temporal gyrus and in the medial temporal. In the present study, inclusion criteria incorporated these different approaches (see table 1 for detailed definitions).
This theory was rekindled in the 1970s, when the first computer-assisted tomography (CT) study showed enlarged lateral ventricles in Schizophrenia [5]. With the introduction of magnetic resonance imaging (MRI) studies, the first MRI study of schizophrenia was conducted in 1984.
Materials and Methods
Magnetic Resonance Imaging Data Acquisition.
First, the diffusion data were corrected for effects of motion and eddy currents through registration of the image relating to the first b0 volume. This was done using the Linear Image Registration Tool SPM (Statistical Parametric Mapping). www.fil.ion.ucl.ac.uk/spm/ package has been designed for the analysis of brain imaging data sequences.
In order to separate brain and no-brain areas, we first generated a brain mask using an automated method in 3Dslicer. (www.slicer.org). Next, the diffusion tensor was estimated for each voxel, by means of weighted with method least-squares in 3D Slicer programming. From these tensor volumes, scalar measures were computed for each voxel, namely FA, AD, SD, and ROI.
Image Preprocessing
All participants were scanned on a 3 T magnetic resonance imaging (MRI) scanner (General Electric, Siemens Medical Solutions, Erlangen, Germany) at Massachusetts General Hospital, Boston, MA, using a standard circular-polarized head coil. DTI was acquired in axial orientation using a diffusion-weighted spin-echo single shot echo-planar imaging sequence (echo time (TE) = 84 ms, repetition time (TR) = 9400 ms, flip angle = 90°, 10 images with b = 0 s/mm2 and 60 no collinear directions with b = 700 s/mm2, 75 contiguous axial slices with 2 mm thickness, field of view (FOV) = 256, matrix size = 128 × 128, resulting in 2 mm isotropic voxels). MRI scans were carefully evaluated to rule out structural abnormalities or artifacts.
Statistical Analysis
The TBSS procedure describes the measurements obtained. In short, FA images from all subjects were coregistered into a template and then linearly aligned into Montreal Neurological Institute (MNI) 152 space. The Johns Hopkins University ICBM-DTI-81 white matter labels atlas24 was used to locate anatomical structures in the MNI152 space. Because our sample included individuals as young as 13 years old that do not match the standard template, a study-specific template was created. The mean FA image of all co-registered subjects then underwent a thinning process to create a white matter skeleton, and subsequently a threshold containing only voxels with FA > 0.2. A search was carried out of the skeleton for neighboring voxels for the maximum local FA values, which were then projected onto the skeleton. Thus, without prior perfect coregistration, the central course of each subject’s fiber tract is represented on the skeleton. To analyze group differences in the other scalars of interest (SD, AD, RD, and ROI), we applied the nonlinear warp obtained from the FA registration, as well as the skeleton projection of the FA data, to the other diffusion scalar volumes.
Results
Our understanding of the neuropathology of schizophrenia and Alzheimer has increased dramatically over the past decade. MRI findings now confirm structural brain abnormalities in schizophrenia and Alzheimer. These findings have widened the scope of both clinical and basic science research and have led to an important research focus on the neurobiology of this disorders. MRI structural findings in schizophrenia include: (1) ventricular enlargement; (2) medial temporal lobe involvement (amygdala, hippocampus and par hippocampal gyrus, (3) parietal lobe involvement (particularly the inferior parietal lobule and its subdivision into angular and supra-marginal gyrus); as well as, (4) the subcortical brain region, including the cerebellum, basal ganglia, corpus callosum, and thalamus. The pattern and number of abnormalities are consistent with a disturbance of connectivity within and between brain regions, most likely neurodevelopmental in origin.
Fractional anisotropy
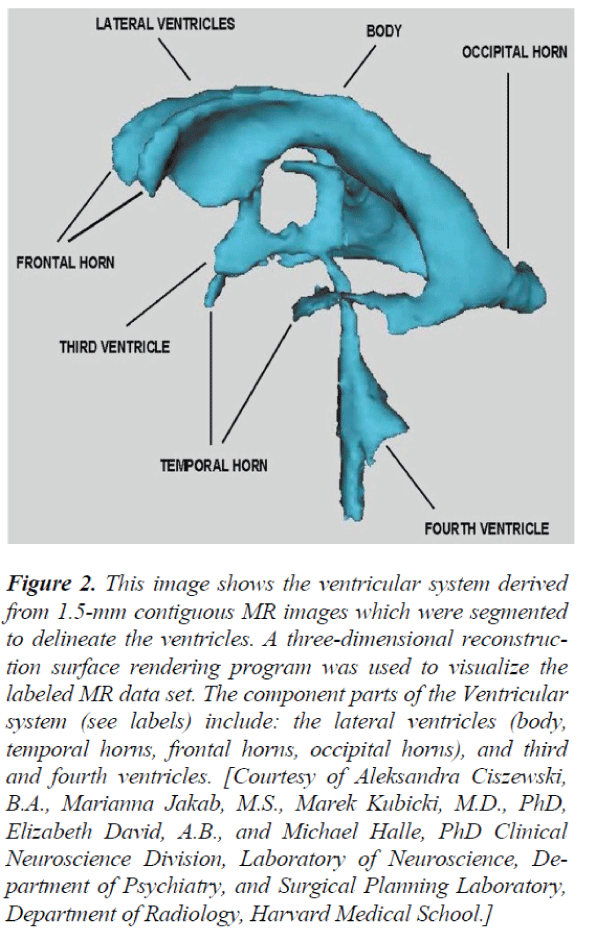
Areas of significantly reduced FA in SD and AD vs controls en ROI are represented in Table 1, where, change was identified predominantly in the parietal-occipital white matter tracts, with relative sparing of the frontal lobes areas. In AD and SD, however, areas of reduced FA in Figure 2, were more widespread and included involvement of the white matter tracts of the frontal lobes. Several studies have reported prefrontal white matter volume Reduction and temporal white matter volume reduction, or a relationship between prefrontal white matter volume reduction and temporal gray matter volume reduction. (Amygdala- hippocampal complex and parahippocampal gyrus)
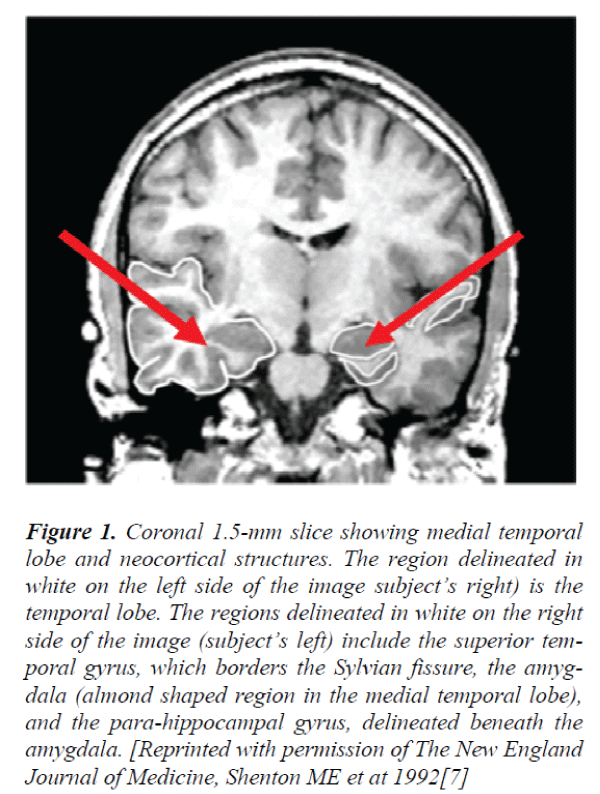
Figure 1. Coronal 1.5-mm slice showing medial temporal lobe and neocortical structures. The region delineated in white on the left side of the image subject’s right) is the temporal lobe. The regions delineated in white on the right side of the image (subject’s left) include the superior temporal gyrus, which borders the Sylvian fissure, the amygdala (almond shaped region in the medial temporal lobe), and the para-hippocampal gyrus, delineated beneath the amygdala. [Reprinted with permission of The New England Journal of Medicine, Shenton ME et at 1992[7]
Figure 2. This image shows the ventricular system derived from 1.5-mm contiguous MR images which were segmented to delineate the ventricles. A three-dimensional reconstruction surface rendering program was used to visualize the labeled MR data set. The component parts of the Ventricular system (see labels) include: the lateral ventricles (body, temporal horns, frontal horns, occipital horns), and third and fourth ventricles. [Courtesy of Aleksandra Ciszewski, B.A., Marianna Jakab, M.S., Marek Kubicki, M.D., PhD, Elizabeth David, A.B., and Michael Halle, PhD Clinical Neuroscience Division, Laboratory of Neuroscience, Department of Psychiatry, and Surgical Planning Laboratory, Department of Radiology, Harvard Medical School.]
Conclusion
MRI findings in schizophrenia include lateral ventricle enlargement, medial temporal lobe volume reduction (including amygdala-hippocampal complex and parahippocampal gyrus), neocortical superior temporal gyrus volume reduction frontal and parietal lobe abnormalities, and subcortical abnormalities affecting the cavum septi pellucidi, basal ganglia, corpus callosum, thalamus, and cerebellum. These findings further suggest the involvement of a large number of functionally related brain regions. Worthy of note here is that, Wernicke [6] and Kraepelin [1] both suggested that schizophrenia could be a disease of insufficient or ineffective communication between these brain regions. This hypothesis has been refueled by recent functional imaging studies, which have demonstrated functional connectivity abnormalities between temporolimbic and prefrontal regions [e.g., refs. 8- 10]. These findings, as well as recent postmortem and genetic studies that demonstrate myelin-related abnormalities in schizophrenia, [12-14] suggest that not only functional, but also anatomical disconnection between brain regions may be involved in schizophrenia. This latter speculation has led to an interest in investigating white matter fiber tract abnormalities in schizophrenia.
Acknowledgements
Acknowledgment and References, the Scientific Medical Institutions: Harvard Medical School (HMS) Psychiatry Neuroimaging Laboratory Dr. Martha Shenton, MA – Boston U.S.A., Institute of Neuro -Psychiatric Research, Clinic Lopez Ibor and Clinical hospital San Carlos department of diagnosis for image, Dr. Juan Arrazola in Madrid - Spain, with contribution in image medicates and in measurements that are applied in different pathologies.
References
- Marek K., Carl-Fredrik W., Robert W. M and Martha S. The application of DTI to Investigate White Matter Abnormalities in Schizophrenia. 2005 December; 1064: 134-148.
- SicongTu, Stephanie W., John R. H., Muireann I., Olivier P., Michael H., lost in spatial translation – A novel tool to objectively assess spatial disorientation in Alzheimer’s disease and fronto temporal dementia. Elsevier Volumen 67, June 2015, Pag. 83-94.
- SMITH RC et al. Nuclear magnetic resonance in schizophrenia: a preliminary study. Psychiatry Res 1984; 12:137-147.
- Martha E. Shenton., Chandlee C. Dikey., Melissa Frumin., Robert W. McCarley. A review of MRI findings in Schizophrenia.Schizophr Res. 2001 April 15; 49(1- 2): 1-52.
- Johnstone EC, et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976; 2: 924-926.
- SMITH RC, et al. Nuclear magnetic resonance in schizophrenia: a preliminary study. Psychiatry Res 1984; 12: 137-147.
- Martha E. Shentonet at 1992. The New England Journalof Medicine.
- Weinberger DR, et al. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149: 890-897.
- Weinberger DR. From neuropathology to neurodevelopment.Lancet 1995; 346: 552-557.
- Shergill SS, et al. mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57: 1033-1038.