ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
The association of chronic myocardial infarction scar detected by DE-MRI with ventricular Remodeling in coronary artery disease
1Department of Radiology, the Second Affiliated Hospital, Kunming Medical University, Kunming, China
2Department of Cardiology, the Second Affiliated Hospital, Kunming Medical University, Kunming, China
- *Corresponding Author:
- Lin Sun
Department of Cardiology
The Second Affiliated Hospital
Kunming Medical University, China
Accepted date: July 25, 2016
Purpose: The aim of this study was to investigate the relationship between myocardial infarction scar and ventricular Remodeling by Delayed-Enhancement Magnetic Resonance Imaging (DE-MRI).
Methods: From June 2009 to May 2013, 20 patients with coronary heart disease who didn’t have a history of acute onset in the last three months were admitted. All patients were diagnosed by DE-MRI. The data including Left Ventricular (LV) Systolic Wall Thickening (WT), Wall Thickening Rate (WTR), Wall Motion Score (WM) and Ejection Fraction (EF) were analysed by Argus software. The myocardial infarction scar score was analysed by DE-MRI according to segment number and transmural extent.
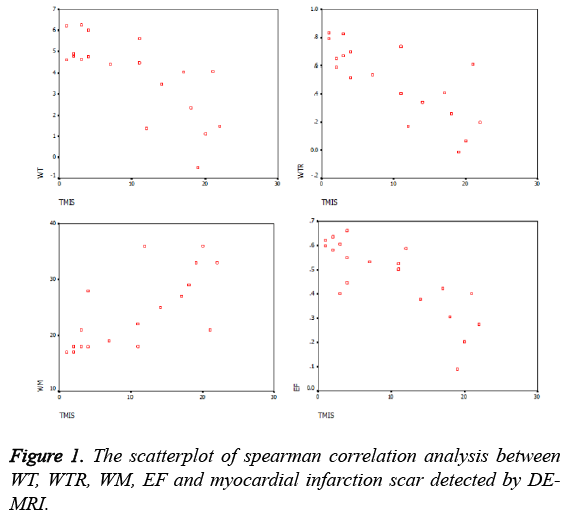
Results: The myocardial infarction scar scored by DE-MRI was negatively correlated with the WT, WTR and EF with a correlation coefficient (R) of -0.788, -0.756 and -0.804, respectively (P<0.05). The myocardial infarction scar scored by DE-MRI was strongly positively correlated with WM with a correlation coefficient (R) of 0.81 (P<0.05).
Conclusion: The myocardial infarction scar score by DE-MRI was negatively correlated with the WT, WTR and EF but scar burden by DE-MRI is strongly and positively correlates with Regional Wall Motion Abnormality (RWMA). Further studies with a larger number of samples are warranted.
Keywords
Myocardial infarction scar, Ventricular remodeling, Delayed enhancement magnetic resonance imaging, Coronary artery disease.
Introduction
Coronary atherosclerosis is a chronic disease with stable and unstable periods and the most common cause of death worldwide. During unstable period, patients may develop a myocardial infarction scar [1]. Myocardial Infarction (MI) scar is a major reason of death and disability for human health. Remodeling was first proposed by Hockman and Bulkley. The concept was applied in the study of scar tissue instead of necrotic tissue for cardiac repair [2]. Until 1990’s, ventricular remodeling was regarded as an important cause of heart failure and the new direction of treatment, which began to attract people's attention. Ventricular remodeling was defined as the structural changes in the left ventricular topography, occurring both acutely and chronically after infarction. There are three major patterns, including concentric remodeling, eccentric hypertrophy and myocardial infarction [3].
Cardiac Magnetic Resonance Imaging (CMR) is an important non-invasive approach for the assessment and risk stratification of patients with MI [4]. It can not only accurately assess Left Ventricular (LV) volumes and function [5] but also detect and quantify the extent of both acute and chronic MI [6,7]. In addition, with high spatial resolution and contrast-to-noise ratio, CMR may allow detailed characterization of infarcts by differentiating the core and peripheral regions [4]. Of the CMR methods, Delayed-Enhancement Magnetic Resonance Imaging (DE-MRI) has been considered as the best imaging method for ventricular remodeling. It is able to assess wall thickening of Left Ventricular Systolic (WT), Ejection Fraction (EF), Wall Motion (WM) and contraction and relaxation of overall or regional quantitatively [8]. All these indicators are valuable for the study of ventricular remodeling. In addition, the advantages of DE-MRI on the observation of myocardial infarction scar have been confirmed by many studies. Ibrahim et al. [9] demonstrated that DE-MRI can detect small myocardial scar which cannot be detected by SPECT. Thus, it is more sensitive than SPECT. Another study also showed that DE-MRI can detect approximate 2 g of myocardial infarct, while the SPECT only can detect 10 g [10]. However, there are few studies on the WT, WM, EF and cardiac function changes detected by DE-MRI [11]. Our study was conducted to investigate the association between myocardial infarction scar detected by DE-MRI and ventricular remodeling.
Materials and Methods
Patients
This cross sectional study was approved by the institutional review board of the Second Affiliated Hospital, Kunming Medical University. Written informed consent was obtained from each subject. Twenty patients (16 men, 4 women, age range: 26-72 years; mean age: 53.3 ± 11.6 years) with coronary heart disease who had no a history of acute onset in the last three months undergoing MRI examination were included from June 2009 to May 2013. The diagnosis was in accordance with the clinical diagnostic criteria of Universal Definition of Myocardial infarction scar, which defined by expert consensus document 2007 European Society of Cardiology (ESC) and the American Heart Association (AHA) jointly.
Patients were excluded if they met the following criteria: (1) Patients with atrial fibrillation or sustained arrhythmias. (2) Patients had medical history or radiographic evidences of other cardiomyopathy or valvular disease. (3) Patients with severe liver or kidney dysfunction caused by liver or kidney organic diseases. The value of serum creatinine was above 2.0 mg.dL-1 and the value of AST or ALT was 5 times more than the upper limit of the normal. (4) Patients with contraindications of MRI, such as body metal implants and claustrophobia. (5) Patients with diagnostic evidence of acute myocardial infarction scar from Electrocardiography (ECG) and clinical biochemistry inspection. (6) Patients could not coordinate MRI examination.
DE-MRI
MRI was performed by using a 1.5 T Siemens Sonata MRI scanner (Siemens Medical Systems, Erlangen, Germany) with an 8-channel phase-array abdominal coil. The ECG signal detected by subcutaneous electrodes was placed in the front of the chest. The gradient field strength was 40 mT/m. The gradient switching rate was 200 mT/m. Left ventricular twochamber long axis, left ventricular short axis, and fourchamber long axis were chosen as the scanning axis positions. Gradient echo sequence was used and 25 images were captured in a cardiac cycle. Scanning parameters were as follows, TR=39.76 ms, TE=1.22 ms, slice thickness= 8 mm and FOV= 276 mm × 340 mm. After the cardiac scanning, a total of 0.2 mmol·kg-1 GD-DTPA was injected twice through the cubital vein by automatic pressure syringe. After the control agent injection, the delay time was 5 ~ 30 min, and delayedenhancement MR images was acquired. The scanning sequence was tfl25tl-psir-segmented with the following parameters: TR=700 ms, TE=4.18 ms, flip angle=25º, thickness=6 mm, gap=0, bandwidth 130 Hz, FOV=325 mm × 400 mm and TI=250 ~ 300 ms.
Observations index and grading criterion
Argus software (Siemens Medical Solutions) was used to calculate left ventricular function parameters, such as systolic wall thickening of Left Ventricular (WT), Wall Thickening Rate (WTR), Wall Motion (WM) and Ejection Fraction (EF). At the same time, according to the information from AHA [12], left ventricle was divided into 17 segments and the WM of every segments was graded 1 ~ 5 semi-quantitatively: 1 score: normal; 2 scores: sports reduction; 3 scores: no movement; 4 scores: contradictory movement; 5 scores: aneurysm formation. Transmural extent of infarction scar was divided into 5 grades, grade 0: normal, recorded as 0 score; grade I: less than 25%, recorded as1 score; grade II: 26% ~ 50%, recorded as 2 score; grade III: 51% ~ 75%, recorded as 3 score; grade IV: 76% ~ 100%, recorded as 4 score. All these parameters were blindly completed by two radiologists.
Statistical analysis
SPSS13.0 software package for Windows (SPSS, Chicago, IL) was used for statistical analysis. Normal distribution data was expressed as means ± SD and non-normal distribution data was expressed as median. Pearson or Spearman's rank test was used to evaluate the association between myocardial infarction scar detected by DE-MRI and ventricular remodeling as appropriate. A P-value of less than 0.05 was considered statistically significant.
Results
The scores of 20 myocardial infarction scar patients, including EF, WM, WT, WTR and DE-MRI were shown in Table 1. The DE-MRI scores were the sum of transmural extent from 17 segments in every patient. The WM scores were the sum of WM from 17 segments in every patient. The EF was calculated from the left ventricular. WT and WTR scores were the mean values of 17 segments in every patient. The correlation of WT, WTR, WM, EF and DE-MRI scores were shown in Table 2. Scatterplots of the correlation analysis among WT, WTR, WM, EF and DE-MRI scores were shown in Figure 1. As shown in the Table 1, Table 2 and Figure 1, spearman rank correlation analysis revealed that there was a negative correlation between the myocardial infarction scar score by DE-MRI and the WT, WTR and EF (R; -0.788, -0.756 and -0.804 respectively; all P<0.05). The myocardial infarction scar score by DE-MRI was strongly positively correlated with WM (R=0.81 and P<0.05): the higher DE-MRI scores, the higher WM and the more obvious WM decreased.
| Index | Values | 95% CI | |
|---|---|---|---|
| Lower | Upper | ||
| EF% | 46.64 ± 15.65 | 39.31 | 53.96 |
| WM | 21.00 ± 10.75 | 20.38 | 26.72 |
| WT(mm) | 4.52 ± 2.23 | 3.06 | 4.80 |
| WTR (%) | 49.05 ± 25.30 | 37.21 | 60.89 |
| DEMRI | 9.00 ± 14.75 | 6.26 | 13.34 |
Table 1: The scores of myocardial infarction scar patients, including EF, WM, WT, WTR and DE-MRI.
| Index | DE-MRI scores of myocardial infarction scar | |
|---|---|---|
| R | P | |
| WT | -0.788 | 0.000 |
| WTR | -0.756 | 0.000 |
| WM | 0.811 | 0.000 |
| EF | -0.804 | 0.000 |
Table 2: The correlation of WT, WTR, WM, EF and DE-MRI scores from 20 myocardial infarction scar patients.
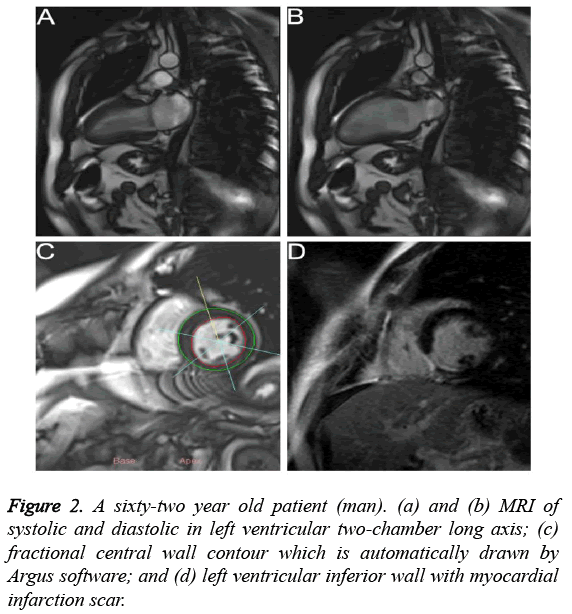
Figures 2a and 2b showed that the MR images of left ventricular two-chamber long axis in the systolic and diastolic stages, respectively. Compared with MR image in the diastolic stage, left ventricular chamber became narrow and the walls became thicken in the systolic stage. As shown in Figure 2c, the fractional central wall contour of left ventricle which was automatically drawn by Argus software indicated that inferior WT became thin and WTR decreased. Figure 2d showed that the left ventricular inferior wall had myocardial infarction scar with a transmural extent >75%, indicating that WT was thinning which was consistent with DE-MRI.
Discussion
Ventricular remodeling is a very complex process and the accurate mechanisms are still unknown. The current common accepted mechanism of post-infarction remodeling is that the process can be divided into two stages: the early stage (72 h or less) and the later stage (>72 h) [13]. After early acute myocardial infarction scar, myocardial cells elongate and deform, which may lead to early ventricular rupture or aneurysm formation [14]. After the formation of myocardial infarction scar, the regional wall becomes stiff, while the elasticity and contractility decrease. In order to maintain a certain amount of blood in the heart to maintain the life activities, the periphery of non-infarcted myocardium is required to increase work burden. Long term of contraction reinforcement will lead to the hypertrophy and deformation of edge and the peripheral edge of non-myocardium. Such changes in the pathophysiology of heart may last for several weeks to months, until the eccentric expansion factors are offset by fibrous scar tension [15]. MRI tagging suggests that myocardial infarction scar remodeling involves not only the infarct zone, but also overall ventricle by [16]. Ventricular remodeling after myocardial infarction scar will directly affect cardiac function by reducing the EF of patient and increasing the incidence of cardiac adverse events [17].
How to effectively control the ventricular remodeling is the key for treating heart failure after myocardial infarction scar. Due to the detected value of Atrial Natriuretic Peptide (ANP) and Brain Natriuretic Peptide (BNP) are correlated with the size of infarct scar and the degree of heart failure, they are used to evaluate heart failure [18]. However, they cannot directly reflect the specific morphology of left ventricular remodeling. Echocardiography is a classical clinical method for visually evaluating ventricular morphological change, which has the advantages of simpleness, fastness, no radiation and low price [19]. However, it has limitation with regard to the subjectivity. In addition, due to the limitation of the window ribs, the probe deviated from the apex and the selection is enslaved to subjective factors of operator. All these impair the accuracy of the measurement of WT and EF [20]. Moreover, echocardiography has other disadvantages such as low spatial image resolution, large measured value and poor reproducibility.
MRI takes advantage of the fast imaging technique. A cardiac cycle can obtain tens of frame images and display a continuous film. The measurement of the cardiac function parameters does not depend on the assumption of the ventricle shape, which is less affected by the operator subjectivity and has high repeatability [21]. All these advantages couple with high spatial resolution and soft tissue resolution, equal cardiac signal and high blood pool signal in sharp contrast, which helps the acquired images provide high-quality information on cardiac anatomy and improve the measurement accuracy of cardiac function, WT and other aspects [22]. A previous report suggested that the detection of cardiac function by MRI and echocardiography has no significant difference, but the EF value of echocardiography is higher than MRI [23]. In addition, MRI may display its "one-stop" superiority that myocardial infarction scar scope, size and transmural extent can be detected simultaneously, especially for small scar and endocardial myocardial infarction scar which are difficult to detect by echocardiography and radioisotope scanning. Therefore, the MRI benefits the study of left ventricular remodeling after myocardial infarction scar.
In this study, myocardial infarction scar integral has a positive correlation with WM: the higher DE-MRI scores, the higher WM, suggesting obvious WM decrease or even reverse movement and aneurysm formation. Previous studies also showed that the thinning of ischemic ventricular wall and the decrease of partial motion had diagnostic value for myocardial infarction scar. When coronary ischemia occurs, segmental WM abnormalities are showed first, which is due to the decrease of coronary blood supply and related regional myocardial contractility. In our study, DE-MRI detection of myocardial infarction scar integral is negatively correlated with WT, WTR and EF: the higher DE-MRI scores, the worse WT and WTR and the lower EF. The mechanism is closely related to left ventricular remodeling. Ischemic myocardium remodeling includes phenotypic change of cardiomyocytes, myocyte death, fibrosis, vascular deficiency, myocardial hypertrophy, lack mitochondria, Ca2+ transport barriers, myosin bradykinesia and non-infarcted myocardial tissue hypertrophy [24]. All these remodeling lead to significant decrease of myocardial contractility, coupled with the role of ventricular pressure, resulting in significant thinning of ventricular wall and decrease of WTR and WM, eventually leading to reducing heart pumping and decreasing EF value.
Ventricular remodeling including the evolution of myocardial infarction scar area, compensatory hypertrophy in surrounding and peripheral zone of myocardial infarction scar area is difficult to diagnose clinically. Early detection of the presence and extent in progress has great value for treatment and prognosis of myocardial infarction scar. Recent studies had shown that DE-MRI can be used as an independent noninvasive imaging method for judging ventricular remodeling in patients. Lund et al. showed that when 24% of the left ventricular infarct size was used to serve as a cut-off value to predict the risk of ventricular remodeling, the sensitivity and specificity were 92% and 93% respectively [25]. Klein et al. suggested that if 20%-25% of the left ventricular wall size had no shrinkage, left ventricular would enlarge [26]. A three-year follow-up study revealed that when the average infarct size of left ventricular wall reached 20% ± 3%, the left ventricular expansion would continue and begin abnormal morphology [27].
Previous animal experiments showed that ventricular remodeling and consequent changes in left ventricular cardiac function had a linear relationship with infarct size [28]. Clinical study also showed that myocardial infarct size, coronary occlusion and other factors can be used as ventricular remodeling predictors [29]. Our study also confirmed that DEMRI detected myocardial infarct scar size and transmural extent have a high correlation with left ventricular remodeling index, which ultimately affected the cardiac function. The WT and WTR directly reflect pathophysiology changes of ventricular wall morphologically. Local WTR abnormalities are the earliest and the most direct expression of myocardial contractile function. Yamashita believed that quantitative analysis of local WTR played an important role in the comprehensive analysis of cardiac function, perfusion, and metabolism of the patients with coronary artery disease [30].
Conclusion
The myocardial infarction scar score by DE-MRI was negatively correlated with the WT, WTR and EF but scar burden by DE-MRI is strongly and positively correlates with Regional Wall Motion Abnormality (RWMA). Further studies with a larger number of samples are warranted.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81260213), Applied Basic Research on Projects in Yunnan Province (2012FB054), Scientific Research Fund Key Projects in Yunnan Province Department of Education (2014Z057).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J 2007; 28: 2525-2538.
- Hochman J, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation 1982; 65: 1446-1450.
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011; 4: 98-108.
- Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006; 114: 32-39.
- Pujadas S, Reddy GP, Weber O, Lee JJ, Higgins CB. MR imaging assessment of cardiac function. J MagnReson Imaging 2004; 19: 789-799.
- Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 2002; 106: 2322-2327.
- Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Muller V. Acute myocardial infarction-evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology 2004; 232: 49-57.
- Yang J, Ma H, Liu J, Wang C, Shi Y, Xie H. Delayed-enhancement magnetic resonance imaging at 3.0T using 0.15mmol/kg of contrast agent for the assessment of chronic myocardial infarction. Eur J Radiol 2014; 83: 778-782.
- Ibrahim T, Bulow HP, Hackl T, Hornke M, Nekolla SG, Breuer M. Diagnostic value of contrast-enhanced magnetic resonance imaging and single-photon emission computed tomography for detection of myocardial necrosis early after acute myocardial infarction. J Am CollCardiol 2007; 49: 208-16.
- Larose E. Below radar-contributions of cardiac magnetic resonance to the understanding of myonecrosis after percutaneous coronary intervention. Circulation 2006; 114: 620-622.
- Themudo RE, Lindahl B, Johansson L, Venge P, Ahlstrom H, Ebeling BC. Unrecognized myocardial scars detected by delayed-enhanced MRI are associated with increased levels of NT-proBNP. Coron Artery Dis 2011; 22: 158-164.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539-542.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications:a consensus paper from an international forum on cardiac remodeling. J Am CollCardiol 2000; 35: 569-582.
- Jain SK, Larsen TR, Darda S, Saba S, David S. A forgotten devil-Rupture of mitral valve papillary muscle. Am J Case Rep 2013; 14: 38-42.
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular Remodeling. Lancet 2006; 367: 356-367.
- Bogaert J, Bosmans H, Maes A, Suetens P, Marchal G, Rademakers FE. Remote myocardial dysfunction after acute anterior myocardial infarction:impact of left ventricular shape on regional function. A magnetic resonance myocardial tagging study. J Am CollCardiol 2000; 35: 1525-1534.
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure:current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011; 4: 98-108.
- Inoue T, Sakuma M, Yaguchi I, Mizoguchi K, Uchida T, Takayanagi K. Early recanalization and plasma brain natriuretic peptide as an indicator of left ventricular function after acute myocardial infarction. Am Heart J 2002; 143: 790-796.
- Wang L, Li G, Wang Z, Liu X, Zhao W. Elevated expression of C3G protein in the peri-infarct myocardium of rats. Med SciMonit Basic Res 2013; 19: 1-5.
- de Simone G, Galderisi M. Quantitation of left ventricular mass and function:balancing evidence with dreams. Ital Heart J 2002; 3: 562-570.
- Karmonik C, Malaty A, Bikram M, Schmitt P, Partovi S, Shah DJ. Fast in vivo quantification of T1 and T2 MRI relaxation times in the myocardium based on inversion recovery SSFP with in vitro validation post Gd-based contrast administration. CardiovascDiagnTher 2014; 4: 88-96.
- Rathi VK, Biederman RW. Imaging of ventricular function by cardiovascular magnetic resonance. CurrCardiol Rep 2004; 6: 55-61.
- Heuschmid M, Rothfuss JK, Schroeder S, Fenchel M, Stauder N, Burgstahler C. Assessment of left ventricular myocardial function using 16-slice multidetector-row computed tomography:comparison with magnetic resonance imaging and echocardiography. EurRadiol 2006; 16: 551-559.
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81: 1161-1172.
- Lund GK, Stork A, Muellerleile K, Barmeyer AA, Bansmann MP, Knefel M. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with re-perfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology 2007; 245: 95-102.
- Klein MD, Herman MV, Gorlin R, Vayo HW. A hemodynamic study of left ventricular aneurysm. Circulation 1967; 35: 614-630.
- Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation 1993; 87: 755-763.
- Fletcher PJ, Pfeffer JM, Pfeffer MA, Braunwald E. Left ventricular diastolic pressure-volume relations in rats with healed myocardial infarction. Effects on systolic function. Circ Res 1981; 49: 618-626.
- Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 2004; 109: 1121-1126.
- Yamashita K, Tamaki N, Yonekura Y, Ohtani H, Magata Y, Nohara R. Regional wall thickening of left ventricle evaluated by gated positron emission tomography in relation to myocardial perfusion and glucose metabolism. J Nucl Med 1991; 32: 679-685.