ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2019) Volume 30, Issue 1
The biocompatibility of resin composite materials on different stem cells.
Hakan Kamalak1*, Ali Taghizadehghalehjoughi2 and Aliye Kamalak3
1Department of Restorative Dentistry, Kahramanmaras Sutcu Imam University, Kahramanmaraş, Turkey
2Atatürk University, Medical Genetic, Erzurum, Turkey
3Department of Endodontics, Kahramanmaras Sutcu Imam University, Elazig, Turkey
- *Corresponding Author:
- Hakan Kamalak
Department of Restorative Dentistry
Kahramanmaras Sutcu Imam University
Turkey
Accepted date: December 17, 2018
DOI: 10.35841/biomedicalresearch.30-18-1162
Visit for more related articles at Biomedical ResearchObjectives: Composite resins should undergo attentive tests to specify their biocompatibility before they have contact with the teeth, regardless of physico-mechanical properties. Hence, this study has been carried out to assess the knowledge of potential cellular responses and biological effects of composite resins on three different cells after two different exposure times.
Methods: The composites Surefil SDR flow, Dentsply Caulk/ABD (SS), X-tra base flowable, Voco Cuxhaven Germany (XB), Venus Bulk Flow, Voco Cuxhaven Germany (VB), Filtek Bulk Flow, 3 M/ESPE-USA (FB), Tetric-Evo bulk flow, Ivoclar Vivadent, Schaan, Liechtenstein (TE) were immersed into molds (Height: 4 mm, Width: 6 mm) and polymerized according to manufacturer's instructions. Human gingival fibroblasts (HGFs), cortical neuron cells (CNCs) and dental pulp stem cells (DPSCs) were inoculated into 24-well plates, separately. Then, the insert membranes with a 3 µm pore width compatible for plates were inserted and dental composite samples were added. The experimental procedures were performed after 24 h and 72 h. The cell viability of the cells was obtained from an MTT assay. Total antioxidant (TAS) and oxidant (TOS) status were examined by using real assay diagnostic kits. The dynamic process of apoptosis analysed by using annexin V-FITC staining with flow cytometry. Human beta defensins were assessed by RT-PCR. Lastly, supernatants were analysed for production of IL6, IL8 and IL10 cytokines.
Results: According to analysis of variance, there were significant differences between three cells. There was an increase in the cytotoxicity values in some groups after 72 h. A 24 h and 72 h experiment revealed that VB was the strongest cytotoxic agent, followed sequentially by XB and FB. Exposure of cells to the composites of SS, XB, VB, FB and TE for 24 h reduced the cell number approximately by 6, 24, 35.6, 28.6 and 1%. A 72 h reduced the cell number for 25.3, 31.6, 40.6, 50.3 and 8%, respectively, by SS, XB, VB, FB and TE.
Conclusions: It can be concluded that from all these data, if the three cell cultures are compared, cortical neuron cells were more sensitive to the resin composites.
Keywords
Anti-oxidant effect, Beta-defensins, Biocompatibility testing, Cell viability, Cells, Composite resins
Introduction
The use of light-cured resin composite materials has recently increased due to their better physico-mechanical properties. The composites used in dentistry consist of different organic molecules. The organic matrix is based on methacrylate chemistry, with the most popular monomers being cross-linking dimethacrylates, for example; bisphenol A-glycidyl methacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), urethane dimethacrylate (UDMA, hydroxyethyl methacrylate (HEMA) or decanediol dimethacrylate (D3MA) [1-5].
Because of this complex structure and the incomplete monomer–polymer conversion, numerous substances such as (co)monomers, additives, or nonpolymerization products are correlated with the soft tissues in the oral cavity and the dentin pulp system. Many of the dental composites can cause cytotoxic effects in the associated tissues with these characteristics [6-12].
In many studies, it was found that resin composite fillings may cause pulp inflammations, genotoxic, mutagenic and estrogenic effects due to extracts from resin composites. Simon reported that the clinical success of dental composite materials depend on their biocompatibility. In this regard, differential release of comonomers such as BisGMA, TEGDMA, HEMA (2- hydroxethyl methacrylate) and UDMA promote the proliferation of the important apoptosis and generation of redox balancedisturbing reactive oxygen species [13].
The biological risk of composites can be partly estimated by cytotoxicity tests in vitro. In general, cell cultures are used for screening and ranking of the cytotoxic potential of dental composites. These researches have been conducted with different primary and permanent cell types using various analysis techniques [14,15].
Because of limited information about the biocompatibility of individual composites, we decided to evaluate the cytotoxic potential of five bulk fill flowable composites in various cells and identify those substances which could pose an particularly high cellular risk. For this reason, we investigated the effect of composite resins on apoptosis, IL-6, IL-8, IL-10 cytokines, cell viability, cellular stress, beta defensins, total antioxidant and oxidant status.
Oxidants play a central role in the pathophysiology of apoptosis via accumulated free radicals. Free radicals cause induced damage to the mitochondria. This situation causes cellular stress, cell integrity, and undesirable tissue damage [16,17].
The Bcl-2 gene expression is known as an important guardian to the apoptotic response. Increase of Bcl-2 value prevents cells from undergoing apoptosis [18]. Thus in this regard, the effect of restorative materials on Bcl-2 gene expression in GFBCs, CNCs and DPSCs was assessed.
Human β-defensins (HBDs) were firstly isolated and characterized based on their antimicrobial activity. HBDs is the first defense line against the microorganisms in the oral cavity and the pulp [19,20]. HBDs are especially effective against gram negative and gram positive bacteria, fungi, and viruses. They also develop a bridge for natural and acquired immunity via the mast cells, immature dendritic cells and T lymphocytes [21-31]. The epithelial cells-like odontoblast cells in the dentin tubules structurally contain HBD-1 and HBD-2 defensins. Stimulation of odontoblast cells with HBD-2 and HBD-1 cause an increase of IL-6 and IL-8 proinflammatory cytokines [32-34]. IL-10 is an anti-inflammatory cytokine that suppresses the secretion of proinflammatory cytokines.
In this research, we focused on the role of different composites on the GFBCs, CNCs and DPSCs. For this reason, we evaluated the nine distinct analysis parameters.
Materials and Methods
Specimen preparation
The restorative materials (Table 1) were placed into standard molds (height: 4 mm, width: 6 mm) and polymerized with LED light device (Elipar Freelight II, 3M-ESPE, (USA/ St.Paul, MN)) according to the instructions of the producing company. After polymerization, cylindrical blocks were obtained. Their edges and surfaces were polished by using the polishing discs (Sof-Lez; 3 M ESPE, (USA/St. Paul, MN). Cylindrical blocks were sterilized under ultraviolet light for 2 h.
| Material Name | Manufacturer | Material Type | Matrix type | Filler Content | Filler content % |
|---|---|---|---|---|---|
| Surefil SDR Flow (SS) | Dentsply Caulk ABD | Bulk Fill Flowable Composite |
BPADMA, TEGDMA UDMA, BHT Dimethacrylate resin |
Silicate glass, silicate oxide, hybrid glass fiber | 80 |
| X-tra Base Flow (XB) |
Voco Cuxhaven Germany | Bulk Fill Flowable Composite |
BisGMA, BISEMA UDMA, Procrylats | Zirconia, silica particles Iterium trifluoride | 75 |
| Venus Bulk Flow (VB) |
Voco Cuxhaven Germany | Bulk Fill Flowable Composite |
UDMA, EPBADMA | Ba-Al-F silicate glass, YbF3, SiO2 | 65 |
| Filtek Bulk Flow (FB) |
3M / ESPE - USA | Bulk Fill Flowable Composite |
UDMA, BisGMA, BISEMA, TEGDMA, Procrylats | YbF3 fillers, zirconium silica particles | 64.5 |
| Tetric-Evo Flow (TE) |
Ivoclar Vivadent Schaan, Liechtenstein | Bulk Fill Flowable Composite |
Dimethacrylate resin | Glass particles prepolymers, iterium trifluoride, mixed oxides | 76 |
Table 1: Materials used in this study.
Preparation of cell culture
Preparation of gingival fibroblast (GFBCs), cortical neuron (CNCs) and dental pulp stem cells (DPSCs): GFBCs and DPSCs were provided by ATCC company (the catalogue number for cell lines: PCS-201-018). CNCs were supplied from Atatürk University Department of Pharmacology. The cells coming in cryofalcons were dissolved in series at normal room temperature and centrifuged for 5 min in a 1200 rpm+4 degrees centrifuge (Bachmann, Germany) by adding 2 μg/ml DMEM medium (Dulbecco’s Modified Eagle’s Medium, Gibco, USA). The settled cells were mixed in the new medium (contains penicillin-streptomycin-amphotericin B) and inoculated in a 25 cm2 flask. When the cells covered 80% of the flask, the passage process was performed by adding 0.4 μg/ml trypsin/EDTA. The cells were taken in a sterile tube and centrifuged for 5 min at 1200 rpm. After the supernatant liquid of the cells was removed, new medium was added and the incubation process was completed by inoculating 250 μl and 105 cells in every well. Then this was kept in an incubator containing 5% CO2 at 37 degrees [35-38].
Preparation of insert membrane well plates
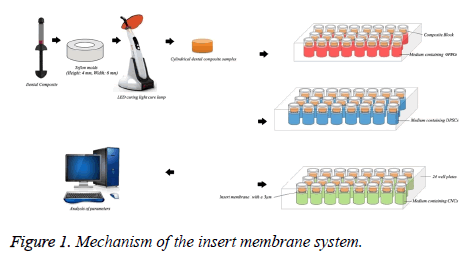
The “barrier test method” used in ISO standards in the evaluation of in vitro cytotoxicity researches and this technique is recommended generally. Dentin tubules serve as a barrier between the material and the pulp. Diameters of the dentinal tubules reach 3-4 μm on the pulp side [39]. Based on this information, insert membrane system was used to simulate the clinical situation (Figure 1).
Determination of Bcl-2, HBD-1, HBD-2, IL-6, IL-8, and IL-10 expression
0.25 μl right and left primary, 0.15 μl probe, 3 μl cDNA, 3 master mixed and 12.75 μl distilled water were added in every strip (tube) and it was adjusted to have a final volume of 20 μl. It was waited at 95 degrees for 60 s and then, a total of 45 cycles were performed at 95 degrees for 10 s. The results were compared with the control group.
RNA isolation
Isolation was performed by using the qiagen RNA isolation kit. 1 μg/ml Qiazole solution was added to the cells in the form of a pellet which were settled in the tube and were kept at room temperature for 5 min. Then, 200 μl chloroform was added, agitated for 15 s, and kept at room temperature for 2-3 min. The samples were centrifuged for 15 min at the temperature of 4 degrees. The colorless fluid at the top was transferred to another tube and it was vortexed by adding 1:1 ethanol alcohol. 700 μl of the sample was taken and placed in a collection tube and then it was closed. The tube was centrifuged at room temperature at 8.000 g for 15 s.
cDNA synthesis
2 μl from the genomic DNA wipeout buffer solution and 1 μg RNasefree water were prepared to have a total volume of 14 μl and then they were kept at 42 degrees for 2 min, after this placed in the ice. 1 μl reverse transcription master mix, Quantrscript RT buffer 5 × 4 μl, 1 μl RT primer mix and 14 μg RNA were mixed and placed in the RT-PCR device. This mixture was heated at 42 degrees for 15 min and at 95 degrees for 3 min and then it was kept up to -20 degrees.
Cytotoxicity analyses
MTT, TAS, and TOS experiments were carried out in order to determine the cell viability and the level of stress factors. Cell fluid was taken from the samples after their interaction with cells for 24 and 72 h and it was kept at -20°C to determine TAS and TOS levels. MTT solution was mixed as 10% with new cell medium and added into 24-well plate in order to determine viability of cells.
MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5- diphenyltetrazolium bromide) assay
The MTT assay is a sensitive and dependable indicator of the cellular metabolic activity. The analysis is based on the reduction of MTT that is a yellow water soluble tetrazolium dye. MTT test was applied at the end of 24 h and 72 h incubation period in the incubator containing 5% CO2 at 37°C. After 4 h MTT incubation, blue formazan crystals (visible within the cell at the optical microscope) were dissolved by adding DMSO (Dimethyl sulfoxide) (sigma, USA). Viable cells with active metabolism convert MTT into a purple colored formazan product with an absorbance maximum near 550 nm wavelength. The absorbance value was read with a spectrophotometer device (μQuant, BadFriedrichshall, Biotek) and the living cell count was obtained. Then we used the following formula to determine the viable cell levels;
Viability (%) ratio=(Sample absorbance value)/(Control group absorbance value) × 100
Principle of the total antioxidant status (TAS) measurement and total oxidant status (TOS)
The total antioxidant and oxidant capacity of the extracts was measured using commercial RelAssayDiagnostics® kits (Turkey). The assessment of TOS assay is done by measuring spectrophotometrically the density of the color related to the amount of oxidants in the sample. The basis of this method bounds up with the oxidation of Fe with valence +2 to an iron complex with valence +3. Fe+3 forms a coloured complex with xylenol orange. Colour intensity differs according to the amount of oxidant in the sample. The absorbance of this colour is measured spectrophotometrically at 530 nm [40,41].
The 2, 2’-azinobis-3-ethylbenzothiazoline-6-sulphonic acid radical (ABTS) reacts with hydrogen peroxide and is oxidised to the ABTS+ molecule in the analysis of TAS assay. The ABTS radical loses its original blue and green colour. The intensity of the colour varies according to the quantity of antioxidants and their antioxidant capacity. The absorbance of this colour is measured spectrophotometrically at 660 nm [40,41]. Then we used the following formula to determine the TOS (μmol H2O2 Equiv./L.) and TAS levels (mmol Trolox Equiv./L).
TOS=Δexample/ΔST2 × 20
TAS =((ΔST1-Δexample))/((ΔST1-ΔST2))
Δ ST2 (ΔStandard 2=ST2 second reading-ST2 first reading), ΔSample (ΔSample=Sample second reading-sample first reading).
Δ ST1 (ΔStandard 1=ST1 second reading-ST1 first reading), Δ ST2 (ΔStandard2=ST2 second reading-ST2 first reading), ΔSample (ΔSample=Sample second reading-Sample first reading).
Statistical analysis
IBM SPSS Statistics 22 (IBM SPSS, Turkey) program was used for the statistical analyses to assess the results obtained in the study. In order to analyse the data, one-way and two-way analyses-of-variance (ANOVA) were performed to detect the significant effects of the variables with the control group. The significance values between the groups were assessed at the levels of p<0.05 and p<0.001 and a significant difference was found between the groups. We used power analysed for established sample size (n=12).
Results
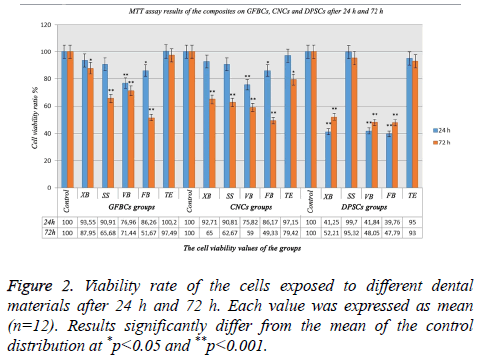
MTT assay results after 24 h and 72 h
The viability ratio of GFBCs was found to be very low in VB and FB after 24 h. In XB, SS and TE; cell viability ratio was high and there was no significant difference between the results. When the viability ratio in the CNCs was assessed; cellular protection was present in SS, XB, and TE groups compared to the control group. Also, the cell viability in FB and VB decreased. Only XB and TE continued cell survival within 24 h. DPSCs playing an important role in the formation of the dentin bridge. The cell viability mostly in TE and SS groups after 24 h were high (Figure 2). After 72 h, the viable cell count was 97% in TE, 87% in XB, 71% in VB, 65% in SS, and 51% in FB. The viability ratio in the CNCs decreased less in TE when compared with the control group. In long-term; XB could not tolerate the viability ratio in 72 h. A decrease of 35% was observed in the viability ratio. The cellular viability in the DPSCs was higher in TE than the control group. This signified that GFBCs were more triggered in TE after 72 h. When the values of SS were observed; cell viability ratios after 24 h and 72 h were almost the same. There was a decrease in cellular viability in almost all groups and an increase in DPSCc after 72h (Figure 2).
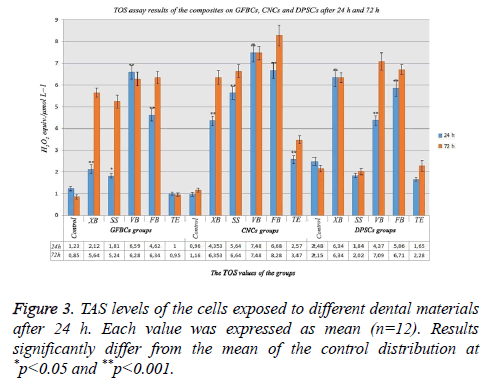
TOS assay results after 24 h and 72 h
When examining TOS analysis results in GFBCs after 24 h, it was found that TOS value was 1.23 in the control group, highest TOS value was obtained in VB and FB, respectively. Antioxidant values increased approximately 6 times in VB, 4 times in FB and 2 times in XB compared with the control group. XB and SS tolerated the antioxidants. When the TOS values in CNCs were examined; it was found that the value in the control group was about 1, oxidative stress increased in XB, SS and TE. Oxidant levels, tolerated because high antioxidant level, suppressed cell stress and degeneration. TOS values were found to be lower in SS and TE than the control group in DPSCs. Normally, TAS and TOS values were compatible with each other. In the present study; antioxidant level increased so much in TE and SS (Figure 3).
When the analysis results in GFBCs were evaluated after 72 h; stress findings increased approximately 6 times than the control group. XB value showed too much increase than the control group. However, when the viability ratio was examined, it was seen as 87%. These results indicated that if the period of the experiment was determined as 96 h or 120 h instead of 72 h, viability ratio in XB would have been lower. The materials other than TE degenerate and eliminate the cells instead of regeneration. When CNCs were examined in the control group, TOS value was found to be 1.16 and a significant increase was observed in the TOS values in other groups. Almost 3 times increase was observed in the oxidant level compared with the control group in the groups other than TE. Antioxidant values increased approximately 3 times in VB, 3 times in FB and 3 times in XB compared with the control group. In other words, this 3-times increase showed that there was excessive stress in the cells. In this study; cellular degeneration was not observed in XB and SS groups; the values were very close to the control group. They were not exposed to any stress. XD, FB and VB showed that there would be an increase in the apoptosis rate. While TOS value in DPSCs was 2.15 in the control group, the highest TOS value was found in VB and FB, respectively (Figure 3).
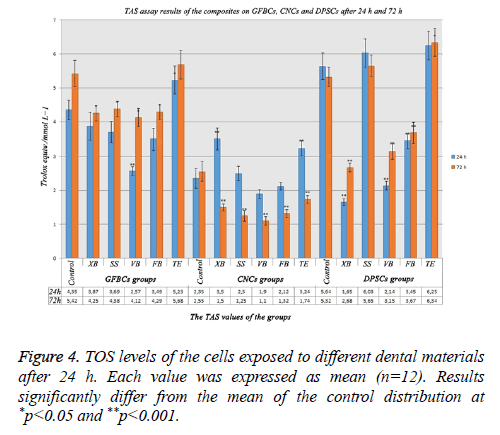
TAS assay results after 24 h and 72 h
The increase in the TAS level signified that cells were protected. When the MTT results in GFBCs were examined, viability values in VB and FB were low. When VB and FB’s antioxidant values were analysed, it was observed that they were very low than the control group. Cells could not protect themselves because cell degeneration was higher in VB and FB. While the antioxidant value was 4.35 in the control group, this value was the lowest in VB group (2.57) after 24 h. TE group had the highest antioxidant value (5.23). While the cells in TE group could protect themselves; the cells in VB, FB, SS, and XB groups could not protect themselves. When total antioxidant values in the CNCs were examined; the antioxidant level decreased in the groups. Protection mechanism was deactivated due to the increase in the cell degeneration. DPSCs showed more sensitive characteristics and required antioxidants could not be produced for the cell protection except for TE and SS groups (Figure 4).
When total antioxidant values in GFBCs were examined after 72 h; the cell degeneration was high in VB and FB. While the antioxidant value was 2.55 in the control group, it was 1.1 in VB group and 1.32 in FB group. This ratio was the lowest in VB group (1.1). When the MTT results in DPSCs were examined after 72 h, the viability values in VB and FB were low. While the antioxidant value was 5.32 in the control group, this value was 3.67 in VB group and 3.15 in FB group. This value was the lowest in XB group (2.68). TE group has the highest antioxidant value (6.34). When the results on the cells were considered, the antioxidant values decreased compared with the control group (Figure 4).
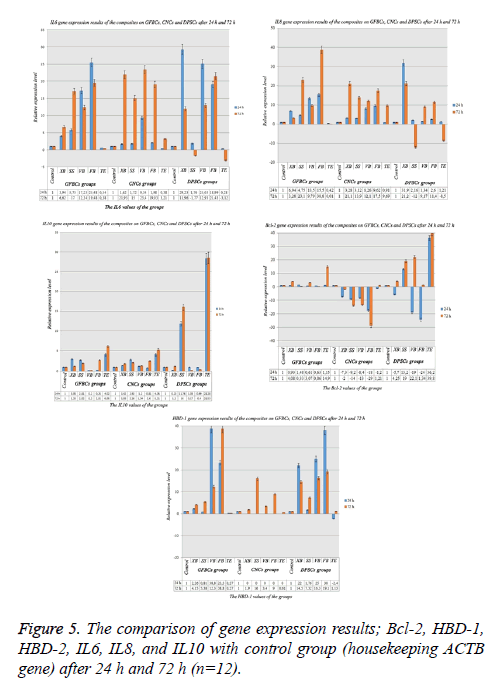
IL-6, IL-8, IL-10, Bcl-2, HBD-1 and HBD-2 gene expression results after 24 h
When the genetic results in DPSCs were assessed, it was found that IL-10, BCL-2, IL-6, HBD-1 and IL-8 were expressed, expressions of HBD-2 could not be taken. After 24 h an increase was observed in VB and FB in terms of the IL-6 values. Also, a similar result was observed for IL-8. An inflammatory process started in VB and FB after 24 h. The lowest IL-6 and IL-8 ratios were observed in TE group. This result showed that the material was biocompatible. In XB and SS; approximately 6-fold increase was in question. Inflammation process started in these materials. However, IL-6 and IL-8 values were balanced with IL-10 in XB and SS. IL-10 values were very low in VB and FB. When the MTT results after 24 h were analysed; it was observed that the cell viability was high in TE, a decrease was observed in cell counts in VB. Similarly, when the BCL-2 values were examined, it was found that this value was 1.15 in TE and it was 0.61 in VB. When the HBD-1 values were assessed, they increased 38 times in VB and 23 times in FB. This increase showed that microbial infection was present. The increase in HBD-1 showed a parallelism with IL-6 and IL-8. However, the HBD-2 gene was not expressed. Expression could not be taken because the materials suppressed on HBD-2 gene. Also, non-expression of HBD-2 showed that the materials had a microbial activity (Figure 5).
When the genetic results in CNCs were examined, it was observed that BCL-2 genes were expressed. Other genes were not expressed. Thus, it may not be correct to decide the cellular death ratios only via TAS and TOS. External experiments are needed to be carried out. Negative genetic results showed that the results fell below normal expression. When the MTT results were examined; degeneration was observed in a great majority of the cells after 24 h and 72 h. This was an indication that cellular degeneration was not associated with apoptosis, but necrosis. It was thought that the cells were directly degenerated before apoptosis. When examining the analysis results; it was found that IL-6 value increased 9 times in VB group and it increased 0.8 times in TE group. The negative decrease in BCL-2 was an indication of exposure to an acute toxicity (Figure 5).
GFBCs are the cells originating from the skin. And they are the important stem cells adjacent to DPSC cells in the teeth. When examining the analysis results, it was observed that IL-6 increase was too much. It increased 29 times compared with the others. There was a serious increase in VB and FB. Similarly, when the IL-8 values were examined, it was observed that they showed a serious increase in XB. Inflammatory factors were high in VB, FB, and XB. IL-10 level that would suppress the inflammatory situation that was found to be low in these groups. The protection mechanism in these groups could not be activated. In other words, cellular degeneration ratios were high. They increased 11 times in SS. When the BCL-2 value was examined; the cells were protected as -5 times in XB, +13 times in SS, -19 times in VB and -24 times in FB. In other words, a great majority of the cells were degenerated in the negative groups. This value increased 36 times in TE group (Figure 5).
IL-6, IL-8, IL-10, Bcl-2, HBD-1 and HBD-2 gene expression results after 72 h
When the genetic results in GFBCs were assessed, it was found that IL-10, BCL-2, IL-6, HBD-1 and IL-8 genes were expressed, expressions of HBD-2 could not be taken. At the end of 72 h, the inflammatory (IL-6) factors showed an increase in XB and SS. IL-6 value which was 3.94 in XB after 24 h and was this value was 6.62 after 72 h. An increase of approximately 2 times was observed. It was seen that these materials started inflammatory reaction in the long term. Additionally, IL-6 values slightly decreased in VB and FB. While they were 17.23 in VB, VB decreased 9.79 after 72 h. These results should support the MTT results. When the MTT results were examined, cell viability ratio in VB decreased from 76% to 71%. It decreased from 86% to 51% in FB. While BCL-2 value was very high in TE, this value was very low in FB. This result showed that while cell viability was high in TE. It was observed that the data were compatible with each other. In this study, IL-10 value increased in TE group, it decreased in VB group. The decrease in HBD-1 caused an increase in proinflammatory cytokines (IL-6 and IL-8). When the analysis results were examined, it was determined that while there was a decrease in the HBD-1 value in TE group compared with control group and an increase was observed in FB. Additionally, IL-6 and IL-8 values of FB were found to be high (Figure 5).
When the genetic results in the CNCs were assessed; it was found that HBD-1, IL-6, IL-10, BCL-2, and IL-8 genes were expressed, HBD-2 expressions could not be taken. There was an increase of 10% in almost all the genes at the end of 72 h. While IL-6 in TE was 0.38 after 24 h, this value was 3.21 after 72 h. Similarly, when the analysis results were assessed; it was found that the IL-6 value in FB was 1.98 after 24 h, it increased to 31.03 after 72 h. When the MTT results were examined, the increase in cellular degeneration over time verified the results of these parameters. While the cell ratio in TE was 97% after 24 h, it was 79% after 72 h. Similarly, while the cell ratio in XB was 92% after 24 h, it was 65% after 72 h. When BCL-2 values were examined, BCL-2 value was found to be positive only in TE group. An increase of 1.21 times was observed. In other groups, negative results were obtained. If the negative value is small, the suppression value of the cells is such high. BCL-2 gene expression value was the highest in FB (-58.79); whereas, it was the lowest in XB group (-2.01). This parameter indicated that cellular death rate is the highest in FB group; whereas, XB group had the lowest cellular death rate among the other suppressed groups. When MTT analysis values were examined in order to verify the result, cell viability ratio was found to be 49% in XB group at the end of 72 h (Figure 5).
When the genetic results in the DPSCs were assessed; it was found that while HBD-1, IL-6, IL-10, BCL-2 and IL-8 genes were expressed, HBD-2 expressions could not be taken. While there was an increase in IL-6 and IL-8 values for the first 24 h, IL-6 and IL-8 suppression was observed in the inflammatory factors in SS and TE groups after 72 h. IL-10 value in SS and TE groups verified the obtained result. While the inflammatory genes were suppressed, the defense genes increased 28 times in TE and 16 times in SS. BCL-2 increased 19 times in SS and 39 times in TE; however, BCL-2 increased in VB (Figure 5).
Discussion
The restorative materials should protect the viability of the pulp. They should not show inflammatory reactions on the application site and should not damage the tissue integrity [17].
Although there are many researches evaluating the biocompatibility of restorative materials, it is not sufficient to investigate the effect of materials on cell viability. Such studies should be supported with different parameters, so that the accuracy of the results increased [1-5]. For this purpose, we compared the probable effect of different dental composites on GFBCs, CNCs and DPSCs. We tested the cells viability, total antioxidant capacity, total oxidant status, apoptosis level, IL-6, IL-8 and IL-10 cytokines, β-defensins: HBD-1 and HBD-2.
The cytotoxic effects of SS and three conventional flowable materials (Venus flow, Filtex Supreme flow and Enamel Plus flow) on DPSCs were analysed with using MTT assays. All the test groups were found cytotoxic [42]. In this study, the cytotoxic effects of composites were assessed on different cells: GFBCs, CNCs and DPSCs and similar results were obtained. It was observed that SS showed low toxicity on DPSCs after 24 h and the cell viability was found as 90.81%. Furthermore, it was determined that bulk-fill flowable composite caused a cell degeneration of approximately 35% after 72 h in the GFBCs. When its effect on DPSCs was examined; it was observed that 0.3% cell death was occurred after first 24 h, then 4.68% cell death was observed at the end of 72 h.
If the formation of free radicals crosses the antioxidant capacity, many metabolic and functional disorders occur. The excess of free radicals threatens molecules such as phospholipids, proteins, DNA and RNA. These molecules provide membrane integrity [43-46]. TAS and TOS are associated with free radicals. Increasing oxidative radicals lead to the reduction of the antioxidant protective mechanism and increase of the oxidative stress. Increased oxidative stress also causes death in the liver cell [47,48]. High TAS value shows that oxidative stress decreases and thus the radicals lose their activity.
In the study assessing the cytotoxic effect of packable and nonpackable dental composites on L-929 murine fibroblasts after 72 h; the composite materials had a toxic effect within the first 24 h and the cytotoxic effect increased over time [49]. On the other hand, in this study, it was determined that TE, XB, SS, VB and FB caused toxic effect in GFBCs and CNCs within the first 24 h. There were a cell death approximately 18% in GFBCs and 25% cell death in CNCs was found after 72 h. A cell regeneration of approximately 10% was observed after 72 h in DPSCs. These different results are due to the organic structure of the filler materials. Especially the different resin monomers cause this situation.
Dommisch et al. have demonstrated that human beta-defensins (hBD-1, -2) play a role in the natural host defense of the human dental pulp. Reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemical analyses were used. In their study, the authors also specified that human β-defensins played an important role in the development of odontoblasts that are responsible for the dentin production [32]. In this study, DPSCs, GFBCs and were used to assess the biological effects. It was observed that HBD-1 and HBD-2 genes were not expressed in the CNCs after 24 h. After 72 h, HBD-1 was expressed but HBD-2 was still not expressed. Similarly HBD-2 was still not expressed in DPSCs and GFBCs after 24 h and 72 h. While the cytotoxic effects of DPSCs, GFBCs and CNCs are being assessed, the studies having an experiment period lasting for more than 72 h can provide more detailed information about the gene expression.
In a study investigating the gene expressions of HBD-1, 2, 3 and 4 in healthy teeth by using reverse transcriptase reaction and real-time polymerase chain reaction; HBD-2 and HBD-3 genes were weakly expressed in healthy and inflammatory pulps [50]. On contrary, HBD-1 and HBD-4 expression significantly increased in inflammation compared to healthy pulps. In their study, Paris et al., found that hBD-1 and hBD-4 played a role in pulpal host defense. In this study, it was observed that HBD-1 and HBD-2 genes were not expressed in the CNCs after 24 h. While HBD-1 gene was expressed after 72 h, HBD-2 gene was still not expressed. When considering the study by Paris et al., assessment of HBD-4 with HBD-1 in the cytotoxicity studies instead of HBD-2 may give more accurate results.
In a study evaluating the cytotoxocity and cytokine expression of silorane and methacrylate-based composite resins; it was proven that cytotoxicity was not observed in siloran-based composites within 6 h and 12 h. Also, the toxic symptoms were seen after 24 h [51]. In this study, there were a cell degeneration of approximately 13% in GFBCs and a cell degeneration of approximately 25% in CNCs after 72 h.
Cytokines are responsible for many pathological situations from the connective tissue breakdown to bone loss [52]. The effect of IL-6 on bone turnover balance was significant, when this balance becomes unbalanced, erosions and bone loss develop. IL-6 increases the collection of osteoclasts over the cells, the number of osteoblasts decreases, bone resorption accelerate and a damaged ossification occurs [53]. The increase of IL-6, inflammation that occurs in systemic diseases, IL-6 is a good marker for determining the toxicity of materials, especially in deeply dentin caries.
As for anti-inflammatory effects, the induction of IL-10 expression was determined. IL-10 plays a role in immune defense in order to avoid multiple cell failure and death. Especially, Tokuda et al. have shown that IL-10 deactivates nuclear factors which result in reduced IL-6 and IL-8 levels in DPSCs [54]. It was specified that the mice with IL-10 deficiency were more sensitive to spontaneous alveolar bone loss and there was an increase in the serum level of type-1 collagen that is a systemic indicator of bone resorption. These results showed that IL-10 is a significant factor in the pathogenesis of alveolar bone loss [55,56].
IL-8 and IL-6 are chemokines causing inflammation. They may extract the inflammatory cells, neutrophils, eosinophils and basophils to the area. BCL-2 gene is an anti-apoptosis gene. In other words, it does not permit cell degeneration due to stress. It protects the cells. HBDs suppress the proinflammatory cytokines, activate the mast cells, and perform degranulation. HBD-1 and HBD-2 show an increase in microbial activation. When these parameters increase, a medium is provided at that area for microbial infection.
In the inflammatory response, IL-8 occurs later compared to other chemotactics. For example, when LTB4 occurs in cell activation within minutes, reaches to the peak point in 3 h and when its concentration starts to decrease, IL-8 is synthesized and secreted and its secretion continues 24 h [57]. IL-8 and other alpha chemokines are found in the blood of the patients having inflammatory reactions and severe traumas and in the inflammation area; they are determined in teeth with an apical abscess, synovial fluid in rheumatoid arthritis, psoriatic skin and in the circulation of the patients having septic shock [58].
When the cytotoxic effect of Filtek™ Z250, Admira and XB were analysed by using undifferentiated pulp cell line, a decrease was observed in the cell viability of murine undifferentiated pulp cells after 14 d [59]. In this study, CNCs were used instead of murine pulp cells. Instead of the direct contact of the cells with restorative materials, the dentinal tubules are simulated and the insert membrane technique was used. In this technique, membranes with 3 micrometer pores were used and thus, the clinical environment was simulated by the experimental study. TE, XB, SS, VB and FB caused a toxic effect on GFBs and CNCs after 24 h in this study. It was observed that there was a cell degeneration of approximately 18% in GFBs and a cell degeneration of 26% in CNCs after 72 h. This result was found different between GFBs and CNCs because of the different production of the intercellular matrix.
Dental pulp has a large neurosensory system that is predominantly provided by the sensorial nerve fibers of trigeminal ganglion. These neurosensory system go ahead from the tooth apex towards the enamel and innervates the surrounding tooth pulp [60-62]. Dense nerve system in the pulp-dentin interface generates the raschkow subodontoblastic plexus and this progress radially throughout the odontoblastic layer [63]. These nerves mediate nociception and form the basis of a toothache. Any problems in neuron cells occur and permanently damage the cell. The number of healthy and viable neuron cells in the pulp is crucial for dental protection, immunosuppression and pulp repair [64]. The assessment of the effect of composite materials on neuronal cells in this study is even more important than DPSCs and GFBCs. Neuron cells cannot repair itself, especially the increase in cell viability count of DPSC cells after 72 h is the biggest indication that these cells repair themselves.
Conclusion
These results give insight into a potential cytotoxic agent on GFBCs, CNCs and DPSCs. Also, we propose a novel mechanism of insert membrane system stimulate the dental tubulus. These findings suggest that neuronal cells were more sensitive to composite materials. These results indicate caution in the usage of VB, FB and TE in restorative dentistry, especially if used near pulp tissue. Especially, FB was highly toxic to these three cells. Opposite, TE was low cytotoxic levels on the cells. All the materials were found to have some toxic effects. This study will be also of outmost importance for the developments of new bioactive restorative materials. Particularly, it may be preferable to use of filler materials free of resin monomers.
Acknowledgement
This research was supported by the authors’ institutions (Firat University Scientific Research Center/Project ID: DHF 16.03).
References
- Six N, Lasfargues JJ, Goldberg M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent 2000; 28: 413-422.
- Modena KC, Casas-Apayco LC, Atta MT, Costa CA, Hebling J, Sipert CR, Navarro MF, Santos CF. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci 2009; 17: 544-554.
- Braonnstram M, Vojinovia O. Response of the dental pulp to invasion of bacteria around three filling materials. ASDC J Dent Child 1976; 43: 83-89.
- Cox CF, Keall CL, Keall HJ, Ostro E, Bergenholtz G. Biocompatibility of surface-sealed dental materials against exposed pulps. J Prosthet Dent 1987; 57: 1-8.
- Grieve AR, Alani A, Saunders WP. The effects on the dental pulp of a composite resin and two dentine bonding agents and associated bacterial microleakage. Int Endod J 1991; 24: 108-118.
- Gerzina TM, Hume WR. Effect of dentine on release of TEGDMA from resin composite in vitro. J Oral Rehabil 1994; 21: 463-468.
- Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil 2001; 28: 1106-1115.
- Geurtsen W, Leyhausen G. Chemical-Biological Interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J Dent Res 2001; 80: 2046-2050.
- Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med 2007; 18: 133-137.
- Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater 2002; 18: 318-323.
- Engelmann J, Leyhausen G, Leibfritz D, Geurtsen W. Metabolic effects of dental resin components in vitro detected by NMR spectroscopy. J Dent Res 2001; 80: 869-875.
- Janke V, von Neuhoff N, Schlegelberger B, Leyhausen G, Geurtsen W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J Dent Res 2003; 82: 814-818.
- Schulz SD, Ruppell C, Tomakidi P. Gene expression analysis of conventional and interactive human gingival cell systems exposed to dental composites. Dent Mater 2015; 31: 1321-1334.
- Hensten-Pettersen A, Helgeland K. Sensitivity of different human cell line in the biologic evaluation of dental resin-based restorative materials. Scand J Dent Res 1981; 89: 102-107.
- Schnitzler P, Wiesenhofer K, Reichling J. Comparative study on the cytotoxicity of different Myrtaceae essential oils on cultured Vero and RC-37 cells. Pharmazie 2008; 63: 830-835.
- Thompson EB. Apoptosis and steroid hormones. Mol Endocrinol 1994; 8: 665-673.
- Composite restorative materials: recommended uses. Council on Dental Materials and Devices. J Am Dent Assoc 1971; 82: 399.
- Berrieman HK, Smith L, OKane SL. The expression of Bcl-2 family proteins differs between nonsmall cell lung carcinoma subtypes. Cancer 2005; 103: 1415-1419.
- Dale BA, Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med 2001; 30: 321-327.
- Harder J, Bartels J, Christophers E, Schröder J-M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 2001; 276: 5707-5713.
- Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol 1995; 13: 61-92.
- Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Ann Rev Microbiol 1995; 49: 277-304.
- Garcia JR, Krause A, Schulz S. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J 2001; 15: 1819-1821.
- Joly S, Maze C, McCray PB, Jr., Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol 2004; 42: 1024-1029.
- Feng Z, Jiang B, Chandra J. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res 2005; 84: 445-450.
- Hazrati E, Galen B, Lu W. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol 2006; 177: 8658-8666.
- Quiñones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003; 17: 39-48.
- Sun L, Finnegan CM, Kish-Catalone T. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol 2005; 79: 14318-14329.
- Chen X, Niyonsaba F, Ushio H. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activates mast cells and increase skin vascular permeability. Eur J Immunol 2007; 37: 434-444.
- Marshall RI. Gingival defensins: linking the innate and adaptive immune responses to dental plaque. Periodontol 2000 2004; 35: 14-20.
- Porter EM, Liu L, Oren A, Anton PA, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun 1997; 65: 2389-2395.
- Dommisch H, Winter J, Açil Y, Dunsche A, Tiemann M, Jepsen S. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol Immunol 2005; 20: 163-166.
- Dommisch H, Winter J, Willebrand C, Eberhard J, Jepsen S. Immune regulatory functions of human beta-defensin-2 in odontoblast-like cells. Int Endod J 2007; 40: 300-307.
- Shiba H, Mouri Y, Komatsuzawa H. Macrophage inflammatory protein-3a and ß-defensin-2 stimulate dentin sialophosphoprotein gene expression in human pulp cells. Biochem Biophys Res Commun 2003; 306: 867-871.
- Kobayashi E, Fujioka-Kobayashi M, Sculean A. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health 2017; 17.
- Jiang ZF, He XP, Liu CH, Yang HY. Effects of the superoxide anionic radicals to the cultured cerebral cortex neuron of newborn rat. Acta Biochimica Et Biophysica Sinica 2001; 33: 552-558.
- Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Methods Mol Biol 2014; 1210: 91-115.
- Kamalak H, Kamalak A, Taghizadehghalehjoughi A3. Cytotoxic effects of new-generation bulk-fill composites on human dental pulp stem cells. Cell Mol Biol (Noisy-le-grand) 2018; 64: 62-71.
- Mjor IA. Dentin permeability: the basis for understanding pulp reactions and adhesive technology. Braz Dent J 2009; 20: 3-16.
- Dikilitas M, Guldur ME, Deryaoglu A, Erel O. Antioxidant and oxidant levels of pepper (Capsicum annuum cv. charlee) infected with pepper mild mottle virus. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2011; 39: 58-63.
- Aksoy L, Kolay E, Agilonu Y, Aslan Z, Kargioglu M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J Biol Sci 2013; 20: 235-239.
- Marigo L, Spagnuolo G, Malara F. Relation between conversion degree and cytotoxicity of a flowable bulk-fill and three conventional flowable resin-composites. Eur Rev Med Pharmacol Sci 2015; 19: 4469-4480.
- Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med 1987; 107: 526-545.
- Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med 1994; 97: 5S-13S; discussion 22S-8S.
- Farinati F, Cardin R, Degan P, De Maria N, Floyd RA, Van Thiel DH, Naccarato R. Oxidative DNA damage in circulating leukocytes occurs as an early event in chronic HCV infection. Free Radic Biol Med 1999; 27: 1284-1291.
- Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med 1991; 91: 2-13.
- Chrobot AM, Szaflarska-Szczepanik A, Drewa G. Antioxidant defense in children with chronic viral hepatitis B and C. Med Sci Monit 2000; 6: 713-718.
- Bolukbas C, Bolukbas FF, Horoz M. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis 2005; 5: 95.
- Franz A, Konig F, Anglmayer M. Cytotoxic effects of packable and nonpackable dental composites. Dent Mater 2003; 19: 382-392.
- Paris S, Wolgin M, Kielbassa AM, Pries A, Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J Endod 2009; 35: 520-523.
- Longo DL, Paula-Silva FW, Faccioli LH. Cytotoxicity and cytokine expression induced by silorane and methacrylate-based composite resins. J Appl Oral Sci 2016; 24: 338-343.
- Johansson A, Claesson R, Hanstrom L, Sandstrom G, Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J Periodont Res 2000; 35: 85-92.
- Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem 2008; 283: 11535-11540.
- Tokuda M, Nagaoka S, Torii M. Interleukin-10 inhibits expression of interleukin-6 and -8 mRNA in human dental pulp cell cultures via nuclear factor-kappaB deactivation. J Endod 2002; 28: 177-180.
- Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res 2003; 82: 632-635.
- Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10: late onset. J Periodont Res 2004; 39: 194-198.
- Bradding P, Roberts J, Britten K. Interleukin-4,-5, and-6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Resp Cell Mol Biol 1994; 10: 471-480.
- Neta R, Oppenheim J, Schreiber R. Role of cytokines (interleukin 1, tumor necrosis factor, and transforming growth factor beta) in natural and lipopolysaccharide-enhanced radioresistance. J Exp Med 1991; 173: 1177-1182.
- Fleming GJ, Awan M, Cooper PR, Sloan AJ. The potential of a resin-composite to be cured to a 4mm depth. Dent Mater 2008; 24: 522-529.
- Rodd HD, Boissonade FM. Comparative immunohistochemical analysis of the peptidergic innervation of human primary and permanent tooth pulp. Arch Oral Biol 2002; 47: 375-385.
- Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech 2003; 60: 503-515.
- Monteiro J, Day P, Duggal M, Morgan C, Rodd H. Pulpal status of human primary teeth with physiological root resorption. Int J Paediatr Dent 2009; 19: 16-25.
- Rodd HD, Boissonade FM. Innervation of human tooth pulp in relation to caries and dentition type. J Dent Res 2001; 80: 389-393.
- Couve E, Osorio R, Schmachtenberg O. Reactionary dentinogenesis and neuroimmune response in dental caries. J Dent Res 2014; 93: 788-793.