ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 1
The correlation between the promotor methylation of TGF-β1 and chemoresistance in epithelial ovarian cancer
Gui-ying Chen1#, Feng-di Hu2#, Jiang-qiong Han3 and Li-hua Yang4*
1Department of Obstetrics and Gynecology, Tai'an Central Hospital of Shandong Province, PR China
2Department of Oncology, the Third Affiliated Hospital of Kunming Medical University (The Tumor Hospital of Yunnan Province), PR China
3Department of Integrative Medicine, the Third Affiliated Hospital of Kunming Medical University (The Tumor Hospital of Yunnan Province), PR China
4Department of Gynaecology, the Second Affiliated Hospital of Kunming Medical University, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Li-hua Yang
Department of Gynaecology
The Second Affiliated Hospital of Kunming Medical University, PR China
Accepted date: October 25, 2017
DOI: 10.4066/biomedicalresearch.29-17-2727
Visit for more related articles at Biomedical ResearchObjective: To investigate the correlation between the promoter methylation of TGF-β1 and chemoresistance in epithelial ovarian cancer, and to explore the therapeutic strategy.
Methods: 68 samples of ovarian cancer and 38 normal ovarian tissues were carried out for analysis of the correlation between the promoter methylation of TGF-β1 and chemoresistance in epithelial ovarian cancer. qRT-PCR and Western blotting assay were performed to detect the expression of TGF-β1 and 5- aza-dc. Demethylation reagent was used to detect the effect of methylation on proliferation of cells with resistance to Paclitaxel (PTX).
Results: By comparing the methylation rate between the sensitive cells and resistant cells, we found statistical difference in SKOV3/TR and SKOV cell lines (94.3% versus 42.9%, χ2=64.43, p<0.01), and in A2780/TR and A2780 cell lines (91.4% and 35.2%, χ2=71.38, p<0.01). However, in other cell lines, no statistical difference was seen. The inhibitory rates in SKOV3/TR (0.19 ± 0.01 μm vs. 0.42 ± 0.02 μm, p<0.01) and A2780 (0.012 ± 0.0001 μm vs. 0.33 ± 0.011 μm, p<0.01) cell lines and cell lines treated by different concentrations (0.01, 0.1, 1 and 10 μm), the inhibitory rate was significantly higher than the control group with a statistically significant difference.
Conclusion: Deficiency of TGF-β1 expression caused by promoter methylation can regulate the adhesion, migration, and apoptosis of cells, thereby being involved in the development and progression of ovarian cancer. With the reversible feature of methylation, it can serve as a therapeutic target in the comprehensive treatment of ovarian cancer.
Keywords
Epithelial ovarian cancer, Chemoresistance, Promoter methylation of TGF-β1
Introduction
Newly-developed cytoreductive surgery and improvement in surgical skills can maximally resect the ovarian cancer. The clinical application of PTX- and cisplatin-based adjuvant chemotherapy also brings possibilities for ovarian cancer treatment. Nevertheless, the overall annual survival rate of ovarian cancer is still dismal [1]. This may be caused by the difficulty in early diagnosis or the primary/secondary drug resistance in chemotherapy. Cisplatin/PTX can serve as the first-line chemotherapeutics in the treatment of advanced ovarian cancer with a response rate of 80% and complete response rate of 40 to 60% [2]. However, a vast majority of patients will suffer from the recurrence within several months after median progression-free survival [3].
Epigenetic variations manifest key roles in development and progression of tumors [4,5]. Highly-methylated CpG island in promotor is the key mechanism inducing the silenced expression or inhibiting the transcription of tumor suppressor [6,7]. Recently, abnormal methylation was found to be closely correlated with the resistance to chemotherapeutics of tumor cells [8,9]. Some studies have reported that abnormal methylation of CpG island in promotor of MCJ (Methylation- Controlled J) can modulate its expressions in normal cells and tumor cells [10]. Methylation in promotor of MCJ can be found in epithelial ovarian cancer tissues in Stage III or IV, suggesting that the high methylation of MCJ is closely associated with the development of chemoresistance and poor prognosis [10]. In addition, Staub et al. [11] found that methylation of HSulf-1 in ovarian cancer decreased expression of HSulf-1 and can lead to the resistance to cisplatin. However, demethylation drugs or HSulf-1-targeted therapy can enhance the sensitivity of cancer cells to chemotherapeutics. Under the normal circumstance, RASSFIA can assist the PTX to disturb the polymerization of spindle body and activate the expression through demethylation, thereby recovering the sensitivity to PTX [8]. However, RASSFIA can also be silenced due to methylation in cancer tissue [8].
In this study, we investigated the methylation in promoter of TGF-β1 in six epithelial ovarian cancer cell lines before and after administration of demethylation drugs. The protein expression of TGF-β1 was measured as well. By these methods, we analysed the correlation between the methylation of TGF-β1 and chemoresistance in epithelial ovarian cancer and explored the potential mechanisms of chemoresistance and therapeutic strategy in epithelial ovarian cancer.
Materials and Methods
Specimens
The specimens of ovarian cancer were preserved in the Pathology Department of Hunan Cancer Hospital between 01/01/2013 and 31/12/2016. We selected a total of 68 cases using random sampling method. These patients aged between 35 and 74 y old with a median of 56 y old. According to the staging criteria of FIGO (2009), there were 20 cases in stage I, 17 in stage II, 21 in stage III, and 10 in stage IV. As for the cell cycles, there were 10 in G1, 12 in G2 and 41 in G3. Regarding the subtypes, there were 25 with serous ovarian adenocarcinoma, 30 with mucous ovarian adenocarcinoma and 13 with endometrioid adenocarcinoma. All these patients had not received any chemotherapy or immunotherapy before the operation. At the same time, we collected a total of 38 normal ovarian tissue samples preserved in the Pathology Department of Hunan Cancer Hospital. Among these patients, they aged between 31 and 70 y old with a median of 55 y old. Ages between the two groups were comparable (p>0.05).
Cells, reagents, and instruments
Rabbit anti-human β-Actin antibody, HRP-labeled goat antirabbit IgG, and HRP-labeled goat anti-mouse IgG antibodies were purchased from Thermo Scientific, US. BCA protein assay kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. RPMI-1640, fetal bovine serum and trypsin was from Life Technologies, US. 5-Aza- CdR, relevant reagents in sulfite treatment, and mouse antihuman TGF-β1 antibodies were from QIAGEN, US. Marker DL-2000 and agarose were from Roche, US. Substances for normalization were from BD, US). Saturated phenol and chloroform were from Biotek, US.
Demethylation
After the cell fusion reached 70% to 80%, cells were washed with PBS twice with the supernatant being discarded. Then, medium supplemented with 5-aza-dc (10 μmol/L) was added into the culture dish with the non-treatment cells as control groups. After 2 d of treatment, the culture medium was discarded and trypsin was used to collect the cells followed by rinsing with PBS twice. After that, cells were collected for extraction of DNA and RNA.
Detection of methylation in cell lines
Reaction condition of Methylation-Specific PCR (MSP) was set as follows: 94°C for 5 min → 95°C for 30 s → appropriate annealing temperature for 45 s → 72°C for 1 min × 40 cycles. The product was loaded in 2% agarose for electrophoresis, and the results were recorded in ultraviolet gel imaging system and analysed. Reaction condition of the sulfite gene sequencing: 94°C for 5 min → 95°C for 30 s → appropriate annealing temperature for 30 s → 72°C for 1 min × 35 cycles → 72°C for 10 min. Product was loaded in the 1.5% agarose for electrophoresis, and results were observed and sequenced.
Real-time quantitative PCR
Ovarian cancer cells that were treated with 5-aza-dc in different concentrations were collected. Using the total RNA extracted from cells as the template, we detected the mRNA expressions of TGF--β1 with Quanti Tect Probe RT-PCR Kit. The expression of GAPDH served as internal reference. This experiment was performed in triplicate, and the average of Ct was used for data statistics.
Western blotting assay
In the sample of total protein, 5X SDS (about 1/5 of the volume of supernatant) was added and boiled for 5 min. After the measurement of protein concentration, 50 μg protein sample was prepared for SDS-PAGE followed by membrane transfer and blocking on a shaker for 1 or 2 h. After the membrane was washed with TBS for 3 times (10 min/time), reagent A and B were well mixed and added on the membrane for 1 to 3 min of reaction at room temperature. Then, PVDF membrane was sealed for exposure in a dark room for 1 min followed by color development and fixation. The results were recorded.
Detection of the effect of 5-aza-dc on proliferation of PTX-resistant cells
MTT assay was applied to detect the cell viability. Ovarian cells collected before and after 5-aza-dc treatment were seeded on a 96-well plate (1 × 104/well) and cultured in an incubator (37°C and 5% CO2) for 24 h. After that, cells were cultured in the medium supplemented with Taxol. To eliminate the influence of the different concentrations of PTX on the effect of 5-aza-dc on cells, cells were treated with four different concentrations: 0.01, 0.1, 1 and 10 μM, and the final volume was adjusted to 200 μL. In the control group, cells were cultured in 200 μL medium without any supplements. After 48 h of culture, cells were incubated in 20 μL MTT solution (5 mg/ml) in each well for 4 h. Then, the culture medium was replaced by 150 μL DMSO, and the violet crystal was dissolved through vibration at a low speed on a shaker for 10 min. Absorbance of each well at wavelength of 490 nm was detected with a microplate reader. IC50 under different concentrations of Taxol was calculated to evaluate cell viability.
Statistical methods
SPSS was used to perform statistical analysis. Calibrated chisquare test or chi-square test was carried out for comparison of enumeration data. t-test was performed for comparison of mRNA expressions before and after drug administration in cell lines. p ≤ 0.05 suggested that the difference had statistical significance.
Results
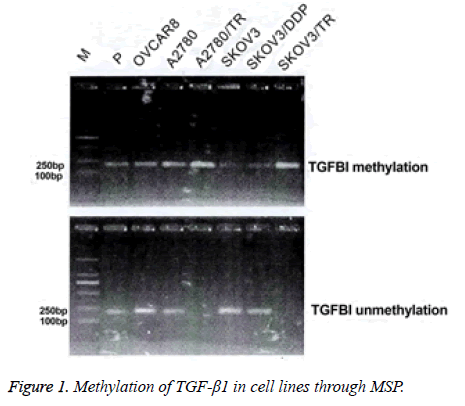
The promoter methylation of TGF-β1 in ovarian cancer cell lines
Complete methylation was seen in SKOV3/TR and A2780/TR cell lines. The other cell lines exhibited partial methylation (Figure 1).
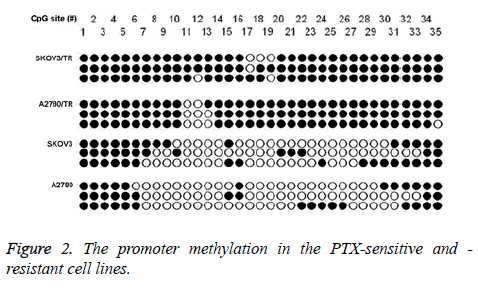
In SKOV3 and A2780 cell lines, partial methylation was identified in the promoter of TGF-β1 (42.9% and 35.2%). Promoter of TGF-β1 in the SKOV3/TR and A2780/TR cell lines exhibited complete methylation (Figure 2).
Comparison of the methylation rate between the PTX-sensitive and -resistant cell lines showed statistical significant differences (SKOV3/TR (94.3%) vs. SKOV3 (42.9%), χ2=64.43, p<0.01; A2780/TR (91.4%) vs. A2780 (35.2%), χ2=71.38, p<0.01).
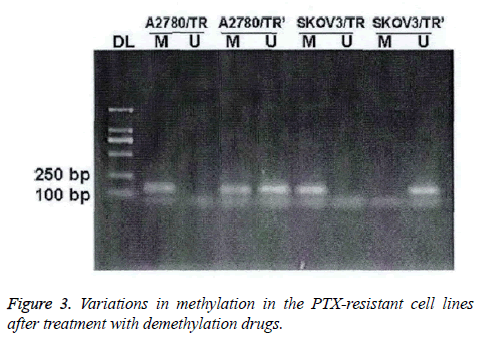
Expression of TGF-β1 in ovarian cancer cells after treatment of demethylation reagent
In PTX-resistant cell lines, TGF-β1 was completely methylated. The methylation could be partially or completely reversed by the treatment of demethylation drugs (Figure 3).
After the treatment of 5-aza-dc, mixed methylation (M+U) was found in the A2780/TR cell lines; while in the SKOV3/TR cell lines, only U and M were eliminated.
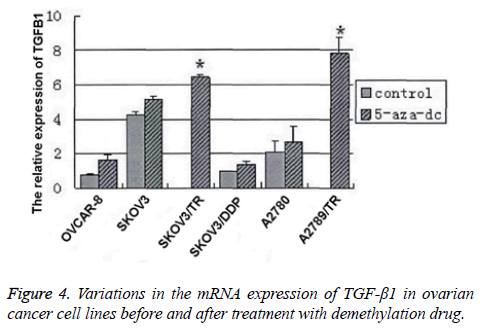
RT-PCR was performed to detect the mRNA expressions of TGF-β1 before and after the treatment. We found that after treatment, the mRNA expression in the PTX-resistant cells was significantly elevated (SKOV3/TR: (7.8 ± 0.9) vs. 0, p<0.01; A2780/TR: (6.4 ± 0.2) vs. 0, p<0.01). However, in other cell lines, the elevations in mRNA expression after treatment showed no statistical significance (OVCAR8: (1.6 ± 0.3) vs. (0.8 ± 0.1), p>0.05; SKOV3: (5.1 ± 0.2) vs. (4.2 ± 0.2), p>0.05; SKOV3/DDP: (1.4 ± 0.9) vs. (0.9 ± 0.2), p>0.05; A2780: (2.7 ± 0.9) vs. (2.1 ± 0.7), p>0.05) (Figure 4). Then, we performed Western blotting assay to detect the protein expression of TGF- β1 in PTX-resistant and -sensitive cell lines before and after treatment. Results were similar to those in RT-PCR. All these results showed that in ovarian cancer cell lines, especially the PTX-resistant cell line, the TGF-β1 expression downregulation is correlated with the promoter methylation.
Proliferation of ovarian cancer cells after the treatment with demethylation reagent
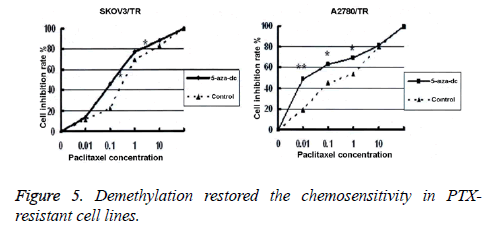
We adopted the MTT assay to detect cell viability in two PTXresistant cell lines, before and after treatment with 5-aza-dc. Results showed that after 96 h of treatment with 5-aza-dc (10 mmol/L), the inhibition rates in SKOV3/TR and A2780 treated with PTX in different concentrations (0.01, 0.1, 1 and 10 μM) were all significantly higher than those in the control group. The differences were statistically significant (SKOV3/TR: (0.19 ± 0.01 μM) vs. (0.42 ± 0.02 μM), p<0.01; A2780: (0.012 ± 0.0001 μM) vs. (0.33 ± 0.011 μM), p<0.01, Figure 5).
Discussion
As one of the malignant tumors with the highest mortality rate in gynecology, ovarian cancer has recently been treated through the cytoreductive surgery in combination with the PTX- and cisplatin-based adjuvant chemotherapy. The comprehensive treatment has gained significant improvement in prognosis of patients. However, the 5 y survival rate remains below 30% in those patients with advanced ovarian cancer. Failure in treatment or recurrence of the patients with advanced ovarian cancer may be cause of the primary or acquired chemoresistance of tumor cells, which is a major challenge in the clinical treatment of ovarian cancer. Thus, the mechanisms leading to the drug-resistance are significant for reversing the drug resistance and improving the efficacy of chemotherapy. Efforts in discovering the way to reverse the drug resistance are of great value in improving the prognosis and increasing the survival rate of patients.
The acquirement of drug resistance in tumor cells is associated with various factors, such as, gene amplification, translocation, deletion or mutation. Epigenetics also plays a key role in the development of drug resistance. As one of the major epigenetic mechanisms, methylation of CpG island in promotor is regarded as an indispensable modification pattern in cells, and also a hotspot in frontier research. Variations of methylation status in tumor cells can give rise to a decrease in overall or local methylation, thereby affecting the sensitivity of tumor cells to chemotherapeutics.
TGF-β1, a kind of extracellular matrix protein, is mainly involved in the adhesion and migration of cells, and a key component in infiltration and metastasis of tumor cells. Also, TGF-β1 can also regulate the proliferation of tumor cells and inhibit the angiogenesis, thus exerting a key effect on the development and progression of tumors. TGF-β1, together with integrin, can mediate the cells to adhere to the extracellular matrix, which is considered as one of the major mechanisms leading to the drug-resistance in tumor cells. Existing studies have reported the role of TGF-β1 in development of drugresistance in malignant tumor cells. It was reported that in NSCLC, TGF-β1 can modulate cell apoptosis, while the deficiency in expression of TGF-β1 results in an increase in resistance of cells to apoptosis, thus promoting the development and progression of tumors, which, however, can be reversed by augmenting the expression of TGF-β1 [3]. Furthermore, Ahmed et al. investigated the mechanism of TGF-β1 in the PTX-resistance in ovarian cancer, and they found that TGF-β1-induced microtubule stabilization can accelerate cell apoptosis, thereby increasing the sensitivity of ovarian cancer cells to PTX [3]. Nevertheless, there remain no studies reporting the correlation between the methylation of promotor in TGF-β1 and the chemoresistance in epithelial ovarian cancer cells.
In this study, we detected the expressions of TGF-β1 and variations in the methylation of promoter in ovarian cancer cell lines (particularly the PTX-sensitive and -resistant cell lines) before and after treatment with 5-aza-dc. Besides, we measured the variations in cell viability in demethylated PTX-resistant cell lines to verify the correlation between the methylation of promotor in TGF-β1 and the PTX-resistance in ovarian cancer cells.
DNA methylation, one of the major epigenetic regulation methods, can vary the gene expression through base modification, instead of altering the sequence. It was reported that methylation in promotor of some genes (apoptosisregulatory genes, DNA damage repair genes, cell cycleregulation genes, cell invasion and adhesion genes and drugtransport genes) can result in the chemoresistance in many malignant tumors (including ovarian cancer). Moreover, genes may be silenced or downregulated under the abnormal methylation of CpG island in promotor in some tumor suppressor genes [3], which might be the major cause for chemoresistance in ovarian cancer patients [3]. The abnormal methylation of these genes can effectively reflect the efficacy and drug-resistance. The expression levels of MCJ in normal cells and tumor cells are regulated by the methylation of promotor. Highly-methylated CpG island in MCJ gene in the advanced ovarian cancer patients may result in the chemoresistance and poor prognosis. Thus, methylation status of MCJ promotor can serve as an epigenetic indicator for efficacy and drug resistance [10]. Latest studies have revealed that MCJ can block the intracellular aggregation of drugs, thereby inducing drug-resistance in tumor cells. This process is believed to be caused by the activation of ABCBI transcriptosome due to the deactivation of hypermethylation in promotor [3]. Besides, in MCJ-deficiency cell lines, resistance to cisplatin or PTX can be reversed when cells are transfected with MCJ gene. Under normal expression, RASSFIA can assist the PTX or any other chemotherapeutics to disturb the polymerization of spindle body, but the abnormal methylation in promotor can silence the gene expression or result in the development of PTX-resistance, which, however, can be restored through the expression [3]. These studies suggest that the abnormal methylation of promotor is closely correlated with the chemoresistance in ovarian cancer.
In this study, we detected the methylation of promotor in TGF- β1 through MSP and BGS in 6 different cell lines: SKOV3/TR (PTX-resistant), SKOVS/DDP (cisplatin-resistant), SKOV3 and A2780/TR (PTX-resistant), A2780 and OVCAR8. The results showed that highly-methylated TGF-β1 was identified in PTX-resistant cell lines, SKOV3/TR and A2780/TR, which is coincident with the deficiency of mRNA expression of TGF- β1 in these two cell lines according to the results of RT-PCR. These data suggested that the methylation is associated with the drug resistance. However, in the remaining 4 cell lines, we also identified the partial expressions of mRNA accompanied with the mixed methylation in promotor of TGF-β1, suggesting that TGF-β1 may be associated with the cell-specificity in SKOV3/TR and A2780/TR cell lines.
Different from the mutation and deletion mechanisms, methylation in promotor can be reversed. This feature provides the possibility for developing the new clinical treatment methods for malignant tumors. Methyltransferase inhibitors not only activate the genes that are silenced by methylation, but also reverse the resistance of ovarian cancer cells to chemotherapeutics. 5-aza-dc, an analogue of cytosine, serves as an effective DNMTs inhibitor in clinical experiment. It can be embedded in the nucleotide, and covalently bind with the active site of DNMTs. This irreversible process can result in the loss of function of DNMTs in cells, thereby eliminating the methylation of DNA and further restoring the gene expression. DNMT inhibitors mainly act on the DNMT1, and the demethylation activity is dependent on the cell replication, in which multiple cell replications can realize the complete demethylation of DNA. In this study, the doubling time of PTX-resistant cell lines was set as 36 to 48 h. So, treatment of 5-aza-dc should last for at least 96 h to fully exert its demethylation effect. According to some studies, the application of 5-aza-dc can restore the expression of hMLHI gene that is silenced by methylation, thus reversing the development of resistance of tumor cells to chemotherapeutics [12-18]. Staub et al. [11] in a study on HSulf-I in ovarian cancer, found that abnormal expression of HSulf-I can induce a decrease in cytotoxicity caused by cobalt drugs, which might be due to the downregulation caused by abnormal methylation in promotor, while the drug resistance can be reversed after the treatment with methyltransferase inhibitor. Here, after treatment with 5-aza-dc, we found that the inhibitory rate of PTX-resistant cell lines was remarkably increased, and the sensitivity of cells to PTX, was significantly enhanced. These results showed that 5-aza-dc could restore the gene expression through demethylation of promotor, thus partially reversing the resistance to PTX. In this study, 40 epithelial ovarian cancer patients underwent follow-up, in which 9 patients who received the PTX-chemotherapy experienced the drug resistance. Samples collected from the corresponding tissues exhibited methylated promoter. Hence, we believed that detection of methylation in promotor of TGF-β1 could serve as the indicator for PTX resistance in epithelial ovarian cancer.
At present, the decrease or deficiency of TGF-β1 expression in a variety of was coincident with the conclusion in this study. This study confirmed that the methylation in TGF-β1 promotor is a frequent event in epithelial ovarian cancer. The methylation in TGF-β1 promoter is associated with the decrease or deficiency of its expression. Thus, we inferred that the abnormal methylation in promoter results in the decrease or deficiency in TGF-β1 expression, thereby regulating cell adhesion, migration and apoptosis, and being involved in the development and progression of ovarian cancer. Besides, a reversible feature of methylation can serve as a new therapeutic target in the comprehensive treatment of ovarian cancer.
References
- Cottrell SE. Molecular diagnostic applications of DNA methylation technology. Clin Biochem 2004; 37: 595-604.
- Ahujia N. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 1997; 58: 3370-3374.
- Wheeler JM, Beck NE, Kim HC. Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predom inantrole of Hmlhi. Proc Nat Acad Sci USA 1999; 96: 10296-10301.
- Yoshikawa H, Matsubrar K, Qian GS. SOCS-I, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28: 29-35.
- Yoon MS, Suh DS, Choi KU. High-throughput DNA hypermethylation profiling in different ovarian epithelial cancer subtypes using universal bead array. Oncol Rep 2010; 24: 917-925.
- Sellar GC, Watt KP, Rabiasz GJ. OPCML at 11q25 is epigenetically inactivated and has umor-suppressor function in epithelial ovarian cancer. Nat Genet 2003; 34: 337-343.
- Balch C, Huang TH, Brown R. The epigenetics of ovarian cancer drug resistance and resensitzation. Am J Obstet Gynecol 2004; 191: 1552-1572
- Tamura G. Hypermethylation of tumor suppressor and tumor-related genes in neoplastic and non-neoplastic gastric epithelia. World J Gastrointest Oncol 2009; 1: 41-46.
- Strathdee G, Vass JK, Oien KA, Siddiqui N, Curto-Garcia J, Brown R. Demethylation of the MCJ gene in stage III/IV epithelial ovarian cancer and response to chemotherapy. Gynecol Oncol 2005; 97: 898-903.
- Liu P, Khurana A, Rattan R, He X, Kalloger S, Dowdy S, Gilks B, Shridhar V. Regulation of HSulf=I expression by variant hepatic nuclear factor I in ovarian cancer. Cancer Res 2009; 69: 4843-4450.
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res 2001; 478: 23-43.
- Burns BS, Edin ML, Lester GE, Tuttle HG Wall ME, Ward MC. Selective drug resistant human osteosarcoma cell lines. Clin Orthop Rel Res 2001; 259-267.
- Ahmed A, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 2007; 12: 514-527.
- Staub J, Chien J, Pan Y, Qian X, Narita K. Epigenetic silencing of HSulf in ovarian cancer: implications in chemoresistance. Oncogene 2007; 26: 4969-4978.
- Watanabe Y, Ueda H, Etoh T, Koike E, Fujinami N. A change in promoter methylation of HMLHI is a cause of acquired resistance to platinum-based chemotherapy in epithelial ovarian cancer. Anticancer Res 2007; 27: 1449-1524.
- Hatle KM, Neveu W, Dienz O, Rymarchyk S, Barrantes R. Methylation-controlled J protein promotes c-Jun degradation to prevent ABCBI transporter expression. Mol Cell Biol 2007; 27: 2952-2966.
- Balch C, Yan P, Craft T, Young S, Skalnik DG. Antimitogenic and chemosensitizing effects of the methylation inhibitor zebularine in ovarian cancer. Mol Cancer Ther 2005; 4: 1505-1514.
- Choi YL, Kang SY, Shin YK, Choi JS, Kim SH. Aberrant hypermethylation of RASSFIA promoter in ovarian borderline tumors and carcinomas. Virchows Arch 2006; 3: 331-336.