ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 13
The effect and its mechanism of Morinda officinalis how polysaccharide on chondrocyte apoptosis induced by IL-1β
Zhang Ru-guo1, Huang Yong-hong2 and Zhang Jian-lin3*
1Department of Orthopedics, the Guizhou Aviation 300 Affiliated Hospital of Zunyi Medical College, PR China
2Department of 3rd Orthopedics and Traumatology, Baoji Traditional Chinese Medicine Hospital, PR China
3Department of 2nd Bone and Joint, Baoji Traditional Chinese Medicine Hospital, PR China
- *Corresponding Author:
- Zhang Jian-lin
Department of 2nd Bone and Joint
Baoji Traditional Chinese Medicine Hospital, PR China
Accepted date: May 22, 2017
Objective: To evaluate the effect and its mechanism of Morinda officinalis how polysaccharide on chondrocyte catabolism and apoptosis induced by IL (Interleukin)-1β.
Materials and Methods: Chondrocytes were randomly divided into 5 groups, control group, IL-1 beta group (50 μg/ml IL-1β in chondrocyte for 24 h), Morinda officinalis how polysaccharide group (2 μg/ml, 5 μg/ml and 10 μg/ml Morinda officinalis how polysaccharide adding to the chondrocyte). The apoptosis rate of the five groups was calculated and the relative expression of Bcl-2 (B Cell Leukemia 2), Bax (Bcl-2 Associated X Protein) and Caspase 9 were quantity by PCR and Western-blot. Elisa kit was used to measure the expression of iNOS in each group.
Results: 50 μg/ml IL-1β can significantly increasing the iNOS and apoptosis rates compared to the control group, and the difference was statistically significant (p<0.05), 10 μg/ml Morinda officinalis how polysaccharide can significantly reduce the chondrocytes apoptosis and iNOS level, the difference was statistically significant (p<0.05). The gene expression and protein expression of Bax and Caspase 9 in IL-1β group was higher than control group, and the Bcl-2 is lower than control group, the differences were statistically significant (p<0.05). Morinda officinalis how polysaccharide in 10 μg/ml can significantly reduce the gene expression and protein expression of Bax and Caspase 9 and down the expression of Bcl-2, the differences were statistically significant (p<0.05).
Conclusion: Morinda officinalis how polysaccharide can increase the expression of Bcl-2, reduce the expression of Bax and Caspase 9, thus can prevent chondrocytes apoptosis induced by IL-1β.
Keywords
Morinda officinalis how polysaccharide, Chondrocyte, Apoptosis
Introduction
Osteoarthritis (OA) is a common degenerative disease with chronic knee pain in clinical practice [1]. The pathological characteristics of OA usually include the damage to the subchondral bone and articular cartilage as well as the secondary apoptosis of chondrocytes [2,3]. Recent studies have shown that OA is mainly characterized as the damaged joint soft tissues caused by the imbalance between the synthesis and degradation of the inflammatory extracellular matrix [4]. Among the most of inflammatory factors, IL-1β, as a kind of proinflammatory cytokines, has been confirmed to play an important role in the occurrence and development of OA [5]. IL-1β, through interrupting the normal metabolism and functions of cartilage by binding with the receptor, can increase the levels of MMP (Matrix Metalloproteinase) (MMP3, MMP9 and MMP13) and iNOs and inhibit the synthesis of protein polysaccharides, leading to a loss of cartilage. Besides, IL-1β can activate the generation of active oxygen to promote the apoptosis of chondrocytes through the Caspase 9 pathway. The intraoperative section of the patients with OA showed the shrunk chondrocytes and formation of apoptotic body. Thus, the apoptosis of chondrocytes is an important link in the pathogenesis of OA [6].
The non-steroid anti-inflammatory drugs have been frequently applied to the treatment of OA, but these drugs are confined in the clinical practices due to the complications such as gastrointestinal bleeding. In recent years, the research focus has been gradually shifted to the traditional Chinese medicine. Polysaccharide from the Morinda officinalis is a kind of monomers extracted from the Morinda officinalis, a kind of traditional Chinese medicine, with various biological activities and distributed widely in the nature [7,8]. Though some studies have confirmed that polysaccharide from the Morinda officinalis can nourish the kidney and strengthen the bones and muscles [9], it still remains unknown in terms of the effect of polysaccharide from the Morinda officinalis on chondrocyte apoptosis induced by IL-1β and the relevant mechanism. Therefore, in this study, the chondrocytes were treated by IL-1β and the polysaccharide from the Morinda officinalis in various concentrations, by which we aimed to investigate the effect of polysaccharide from the Morinda officinalis on the chondrocyte apoptosis and the relevant potential mechanism.
Materials and Methods
Experiment reagents
5 male SD rats aging 8 weeks (Qualification Number: SCXK (Yu 2002008)) with the weight ranging from 180 to 200 g were provided by the Animal Experiment Center of the Medical School; polysaccharide from the Morinda officinalis (Dalian Meilun Biology Technology Co., Ltd, China); IL-1β (Peprotech, USA); DMEM/F12 medium, PBS, calf serum, pancreatic enzyme (Gibco, USA); AnnexinV-FITC apoptosis detection kit, mouse anti-rat Bax, Bcl-2 and Caspase 9 primary antibody (Abcam, USA); RIPA, PMSF, goat anti-mouse secondary antibody and ECL (Boster, China); ReverTra Ace qPCR RT-PCR detection kit (Takara, Japan); SYBR® Green Realtime PCR Master Mix quantitative detection kit (Takara, Japan); iNOS Elisa kit (Shanghai Enzyme-linked Biotechnology Co., Ltd, China). This study had been approved by the ethic committee of the college.
Extraction and grouping of the chondrocytes
SD rats were executed by dislocating the cervical vertebra and disinfected by iodine and alcohol. Then, the knee joint was exposed through the anterior part of the knee and the cartilage on the surface of the joint was scraped carefully with a knife. The obtained cartilage was cut into pieces by scissors, in which 0.25% trypsin was added to isolate the chondrocytes using enzyme digestion for culture. After the primary cells were isolated, culture of the rest cells was continued in the incubator (37°C, 5% CO2, saturated humidity) with the addition of the DMEM/F12 medium containing 10% calf serum. The medium was replaced after 1 w of culture and later every 2 or 3 d. Till the cell confluence reached 80 to 90%, cells were subcultured. In the 2nd generation, we identified the chondrocytes by staining using alcian blue and type II collagen. The intensity of cells in the 3rd generation, being starved overnight, was adjusted to 3 ×105/ml by digestion and divided into 5 groups randomly, i.e. the control group (no intervention), IL-1β group (treated by 50 μg/ml of IL-1β for 24 h) and 3 polysaccharide from the Morinda officinalis groups which were treated by 2 μg/ml, 5 μg/ml and 10 μg/ml of polysaccharide from the Morinda officinalis was added into each group respectively with the addition of 50 μg/ml of IL-1β. 5 duplications were set in each group and the experiment was repeated for 3 times.
Detecting the influence of polysaccharide from the Morinda Officinalis on the apoptosis of chondrocyte induced by IL-1β in rats using flow cytometry
AnnexinV-FITC apoptosis detection kit was used for detecting the apoptosis rate of the cells in each group. After being treated by IL-1β for 24 h, the culture dishes were taken out. The digestive enzyme was added into the cells, followed by the addition of NB serum to terminate the digestion. Then the digested cells were transferred into the tubes for centrifugation at 1000 g for 5 m. Cell resuspension solution was prepared using PBS (pH 7.4 to 7.6) and the cell counting was performed. In the extracted 5 × 104 cells, we added 500 μl of AnnexinVFITC and then 5 μl of propidium iodide and mixed well. After 30 min of incubation in the shady place, the flow cytometry was used to detect the cell apoptosis rate in each group.
Detecting the protein expression of Bcl, Bax2 and Caspase 9 using PCR
The culture dishes were taken out for extraction of total RNA using Trizol. According to the instruction of ReverTra Ace qPCR reverse transcription kit, cDNA was prepared by reverse transcription of the extracted total RNA. The primer of target molecules was detected as follow: For Bcl-2, upstream 5’-CAA ACT GCT AAA TGA CGA GG-3’, and downstream 5’-GGG AAA GGT TGT GTA GGG TC-3’; for Bax, upstream 5’- AGATCCTGACCGAGCGTGGC-3’, and downstream 5’-CCA GGG AGG AAG AGG ATG CG-3’; for Caspase 9, upstream 5’-AGATCCTGACCGAGCGTGGC-3’, and downstream 5’- CCA GGG AGG AAG AGG ATG CG-3’; for β-actin, upstream 5’-AGATCCTGACCGAGCGTGGC-3’, and downstream 5’-CCA GGG AGG AAG AGG ATG CG-3’. QRT-PCR was performed using SYBR® Green Realtime PCR Master Mix quantitative detection kit. The semi-quantitative calculation was carried out in the formula of 2-ΔΔCt with the β- actin as internal reference.
Detecting the expression of relevant protein using Western blot
The cells in the culture plates were treated by 3 ml/g protein lysate for sufficient lysis then centrifuged and the proteins were collected. The concentration of total protein was assayed using BCA. Thereafter, the same amount of protein was added into the same volume of 2XSDS loading buffer and heated to boil for 10 min, followed by SDS-PAGE, membrane transfer, blocking, incubation using primary antibody overnight (1:150), and incubation using secondary antibody (1:5000) at 37°C for 2.5 h. Then the ECL was added for developing the image in the gel-imaging system. Gray value was calculated using Image J software. Relative gray value=(Detected gray value of protein/ GAPDH gray value for internal reference) ×100%.
Detecting the iNOS expression using Elisa
After 24 h of treatment, the cells were extracted for dilution with standard diluent according to the instruction of Elisa kit. Stop buffer was added into the loaded samples, mixed well, and then OD value was read at 450 nm of wavelength on the microplate reader. The activity of iNOS was calculated by the OD value.
Statistical analysis
SPSS20.0 was applied to the statistical analysis. All data were presented as mean ± standard variation. Single-way variance analysis was performed in intergroup comparison. Dunnet-t test was performed in pairwise comparison. Significant level was set as p=0.05.
Results
Comparison of the apoptosis rates in each group
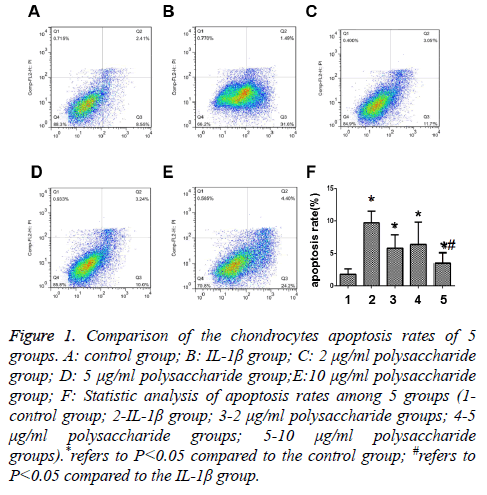
The results showed that the apoptosis rate of chondrocytes after 24 h of treatment by 50 μg/ml IL-1β was significantly higher than that in the control group with statistically significant differences (t=-6.721; p<0.05) (Figures 1A and 1B), and that significant decrease was seen in the apoptosis rate after the cells were treated by 10 μg/ml polysaccharide from the Morinda officinalis with statistically significant differences (t=-4.287; p<0.05) (Figure 1E), and there was no statistically significant difference in comparison of the apoptosis rates among the groups treated by 2 μg/ml or 5 μg/ml polysaccharide from the Morinda officinalis and the IL-1β group (t=-1.976; p>0.05; t=-1.261; p>0.05) (Figures 1C and 1D).
Figure 1: Comparison of the chondrocytes apoptosis rates of 5 groups. A: control group; B: IL-1β group; C: 2 μg/ml polysaccharide group; D: 5 μg/ml polysaccharide group;E:10 μg/ml polysaccharide group; F: Statistic analysis of apoptosis rates among 5 groups (1- control group; 2-IL-1β group; 3-2 μg/ml polysaccharide groups; 4-5 μg/ml polysaccharide groups; 5-10 μg/ml polysaccharide groups).*refers to P<0.05 compared to the control group; #refers to P<0.05 compared to the IL-1β group.
The expression of Bcl, Bax2 and Caspase 9 in each group
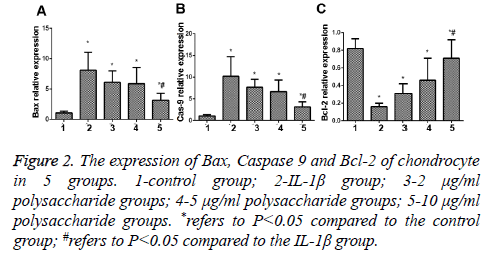
The results of PCR indicated that the expression of Bax and Caspase 9, the apoptosis genes of chondrocytes, was significantly increased (Figures 2A and 2B), while the expression of Bcl-2, the anti-apoptosis gene, was remarkably decreased, and the difference between the IL-1β group and the control group was statistically significant (t=0.238; p<0.05) (Figure 2C); in comparison with the IL-1β group, significant decreases were seen in expression of Bax and Caspase 9 but the expression of Bcl-2, the anti-apoptosis gene, was remarkably increased in the group treated by 10 μg/ml polysaccharide from the Morinda officinalis with statistically significant differences (t=0.101; p<0.05).
Figure 2: The expression of Bax, Caspase 9 and Bcl-2 of chondrocyte in 5 groups. 1-control group; 2-IL-1β group; 3-2 μg/ml polysaccharide groups; 4-5 μg/ml polysaccharide groups; 5-10 μg/ml polysaccharide groups. *refers to P<0.05 compared to the control group; #refers to P<0.05 compared to the IL-1β group.
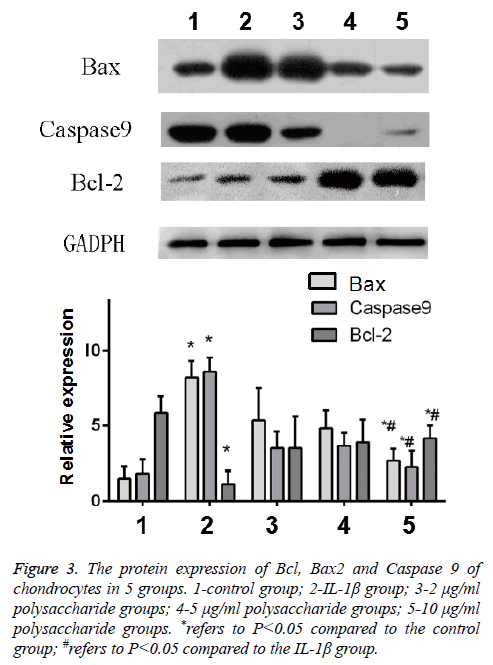
The results of Western-blot also indicated that after the cells were treated by IL-1β, significant increases were found in the expression of Bax and Caspase 9, the apoptosis protein of chondrocytes, but the expression of Bcl-2, the anti-apoptosis protein, was obviously decreased, and the difference was statistically significant (t=-4.397; p<0.05); in comparison with the IL-1β group, significant decreases were seen in the protein content of Bax and Caspase 9 but the protein content of Bcl-2 was remarkably increased in the group treated by 10 μg/ml polysaccharide from the Morinda officinalis with statistically significant differences (t=-3.747; p<0.05, Figure 3).
Figure 3: The protein expression of Bcl, Bax2 and Caspase 9 of chondrocytes in 5 groups. 1-control group; 2-IL-1β group; 3-2 μg/ml polysaccharide groups; 4-5 μg/ml polysaccharide groups; 5-10 μg/ml polysaccharide groups. *refers to P<0.05 compared to the control group; #refers to P<0.05 compared to the IL-1β group.
Comparison of the iNOS expression in each group
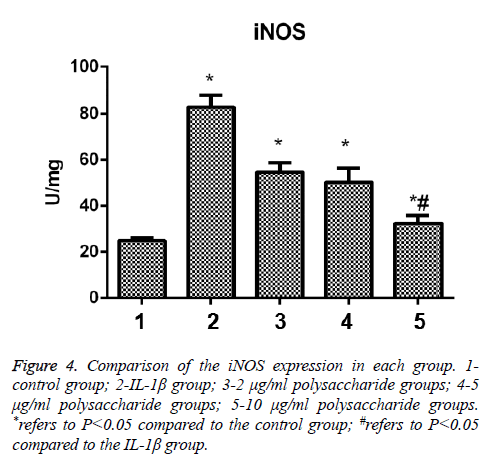
After the cells were treated by IL-1β, the expression of iNOS of chondrocytes was significantly increased, and the difference was statistically significant in comparison between the control group and the IL-1β group (t=-12.286; p<0.05); in comparison with the IL-1β group, significant decrease were seen in the expression of iNOS in the group treated by 10 μg/ml polysaccharide from the Morinda officinalis with statistically significant differences (t=-9.949; p<0.05, Figure 4).
Discussion
In this study, we, for the first time, investigated the influences of polysaccharide from the Morinda officinalis on the chondrocyte apoptosis by establishing the chondrocyte apoptosis model using 50 μg/ml IL-1β and found the decreases in the apoptosis rates of chondrocytes after the chondrocytes were treated with polysaccharide from the Morinda officinalis in various concentrations, in which the effect of 10 μg/ml polysaccharide from the Morinda officinalis was the most significant. Moreover, we found that polysaccharide from the Morinda officinalis might exert the anti-apoptosis effect through the Bax/Bcl-2 pathway in the further study of the expression of the apoptosis-related genes and protein in the chondrocytes.
Polysaccharide from the Morinda officinalis is a kind of monomer extracted from the traditional Chinese medicine with various biological activities [10]. Mengyong et al. [11] performed intervention treatment with polysaccharide from the Morinda officinalis for the rat OA model and found that polysaccharide from the Morinda officinalis can decrease the level of MMP13 in the cartilage of knee joint, suppress the degradation of extracellular matrix and delay the onset of OA. Apoptosis is also called the programmed cell death. In this research, we, according to the previous literature, added the 50 μg/ml IL-1β into the chondrocyte and found that the apoptosis rate of chondrocyte was increased by 5 times, which is coincident with the conclusion of previous literatures [12,13]. Some studies reported that polysaccharide from the Morinda officinalis can regulate the expression of MMP-13 to delay the onset of degeneration of articular cartilage by suppressing the NF-kB signal pathway to inhibit the expression of p65 in a dose-dependent manner. Similar results were also obtained in this study, i.e. 10 μg/ml polysaccharide from the Morinda officinalis could obviously suppress the apoptosis rate of chondrocyte induced by IL-1β, but there was no statistically significant difference in comparison of the apoptosis rates among the groups treated by 2 μg/ml and 5 μg/ml polysaccharide from the Morinda officinalis and the group treated by 50 μg/ml IL-1β, suggesting that a high dosage of polysaccharide from the Morinda officinalis can exert the antiapoptosis effect to protect the chondrocyte.
In this study, we also identified the apoptotic chondrocytes in each stage using AnnexinV-FITC/PI double staining method and found that gene expression and protein expression of Bax and Caspase 9 in IL-1β group was higher than control group, and the Bcl-2 is lower than control group. Morinda officinalis how polysaccharide in 10 μg/ml can significantly reduce the gene expression and protein expression of Bax and Caspase 9 and down the expression of Bcl-2, polysaccharide from the Morinda officinalis could significantly reduce the apoptosis of chondrocytes. Cell apoptosis refers that a series of intracellular signal transductions are initiated by the stimulation of various signals, finally leading to the generation of apoptosis body, resulting in the death of cell [14]. However, Bcl-2 and Bax belong to the family of Bcl-2, including Bax, the apoptotic molecule, and Bcl-2, the anti-apoptotic molecule [15]. All these two molecules have been confirmed to be associated with the mitochondria apoptosis through influencing the distribution of membrane potentials of mitochondria to trigger the apoptosis of cell [16]. Bcl-2 can enhance the resistance of the cells: increased expression of Bcl-2 would lead to the formation of homodimer to suppress the apoptosis of cells [17]; while the excessive expression of homologous Bax would also lead to the formation Bac/Bax homodimer to facilitate the apoptosis of cells [18]. In this study, we found that the Bax in the chondrocyte was significantly increased after the chondrocyte was treated by IL-1β, indicating that IL-1β might influence the cell apoptosis by affecting the level of Bax in the chondrocyte, and the application of polysaccharide from the Morinda officinalis could suppress the expression of Bax, and then the expression of downstream Caspase 9 to delay the onset of OA.
In the results of detecting the iNOS levels in chondrocytes in the 5 groups, we found that 50 μg/ml IL-1β can significantly increasing the iNOS and apoptosis rates compared to the control group, we identified a significantly increased level of iNOS which could suppress the synthesis of the chondrocyte matrix and type II collagen, forming a micro-environment in chondrocyte which was not fit for the survival of chondrocyte, further facilitating the apoptosis of chondrocytes. Polysaccharide from the Morinda officinalis can resist the catabolism of chondrocyte through reducing the NO level by decreasing the level of iNOS.
In conclusion, polysaccharide from the Morinda officinalis could delay the progression of OA by inhibiting the apoptosis of chondrocyte induced by IL-1β through reducing the expression of Bax and Bcl-2. However, there remain no relevant in-vivo studies to further verify the best dosage.
References
- Israel HA. Internal Derangement of the Temporomandibular Joint: New Perspectives on an Old Problem. Oral Maxillofac Surg Clin North Am 2016; 28: 313-333.
- Bang D, Xu J, Keenan R, Pike V, Lehmann R. Cardiovascular disease prevalence in patients with osteoarthritis, gout, or both. Bull Hosp Jt Dis 2016; 74: 113-118.
- Cho SD, Youm YS, Kim JH. Patterns and influencing factors of medial meniscus tears in varus knee osteoarthritis. Knee Surg Relat Res 2016; 28: 142-146.
- Oh WS, Lee YS, Kim BK. Posterior shift of contact point between femoral component and polyethylene in the LCS rotating platform implant under-weight bearing condition. Knee Surg Relat Res 2016; 28: 137-141.
- Fu D, Lu J, Yang S. Oleic/palmitate induces apoptosis in human articular chondrocytes via upregulation of NOX4 expression and ROS production. Ann Clin Lab Sci 2016; 46: 353-359.
- Zhou K, Hu L, Liao W, Yin D, Rui F. Coptisine prevented IL-1β-induced expression of inflammatory mediators in chondrocytes. Inflammation 2016; 39: 1558-1565.
- Liang Y, Duan L, Xiong J. E2 regulates MMP-13 via targeting miR-140 in IL-1beta-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther 2016; 18: 105.
- Duan C, Chen K, Yang G, Li T, Liu L. HIF-1a regulates Cx40-dependent vasodilatation following hemorrhagic shock in rats. Am J Transl Res 2017; 9: 1277-1286.
- Zhi K, Li M, Bai J. Quercitrin treatment protects endothelial progenitor cells from oxidative damage via inducing autophagy through extracellular signal-regulated kinase. Angiogenesis 2016; 19: 311-324.
- Hou KJ, Lin CJ, Chen C . Association of probiotics and bone mineral density in Chinese patients with type 2 diabetes. Biomedical Research India 2017; 28: 129-133.
- Mengyong Z, Caijiao W, Yong G. Extraction, characterization of polysaccharides from Morinda officinalis and its antioxidant activities. Carbohydrate Polymers 2009; 78: 497-501.
- Chien SY, Huang CY, Tsai CH. Interleukin-1beta induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clin Sci (Lond) 2016; 130: 667-681.
- Xie J, Fu N, Cai LY. The effects of interleukin-1beta in modulating osteoclast-conditioned mediums influence on gelatinases in chondrocytes through mitogen-activated protein kinases. Int J Oral Sci 2015; 7: 220-231.
- Sun WZ, Li MH, Chu M, Wei LL, Bi MY. Id1 knockdown induces the apoptosis and inhibits the proliferation and invasion of ovarian cancer cells. Eur Rev Med Pharmacol Sci 2016; 20: 2812-2818.
- Zhang M, Zhang SQ, Hui Q, Lei L, Du XL, Gao WW, Zhang RH, Liu GW, Li XB, Li XW. β-Hydroxybutyrate facilitates fatty acids synthesis mediated by sterol regulatory element-binding protein1 in bovine mammary epithelial cells. Cell Physiol Biochem 2015; 37: 2115-2124.
- Gao S, Tan H, Zhu N. Oridonin induces apoptosis through the mitochondrial pathway in human gastric cancer SGC-7901 cells. Int J Oncol 2016; 48: 2453-2460.
- Li HG, Chen JX, Xiong JH. Myricetin exhibits anti-glioma potential by inducing mitochondrial-mediated apoptosis, cell cycle arrest, inhibition of cell migration and ROS generation. J Buon 2016; 21: 182-190.
- Kim WK, Pyee Y, Chung HJ, Park HJ, Hong JY. Antitumor activity of spicatoside A by modulation of autophagy and apoptosis in human colorectal cancer cells. J Nat Prod 2016; 79: 1097-1104.