ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2006) Volume 17, Issue 1
The effects of biopsy and cryopreservation by vitrification on the in vitro development of eight-cell mouse embryos
1Institute of Medical Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selan-gor, Malaysia
2School of Biological Sciences and Agriculture, Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
3Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
4Institute of Biological Sciences, Faculty of Science, University of Malaya 50603, Kuala Lumpur, Malaysia
- *Corresponding Author:
- Nor Ashikin Mohamed Noor Khan
Institute of Medical Biotechnology, Faculty of Medicine
Universiti Teknologi MARA 40450 Shah Alam, Selangor, MALAYSIA
Phone: +603-55442986
Fax: +603-55435530
E-mail: noras011 ( at ) salam.uitm.edu.my
Accepted date: February 14, 2006
In vitro and in vivo development of cryopreserved and non-cryopreserved biopsied 8-cell mouse embryos were examined in this study. It was observed that intact and biopsied em-bryos could successfully be cryopreserved by using the blastomere biopsy and vitrification procedure. However, in vitro survivability of the embryos declined steadily with advancing developmental stages, irrespective of the treatments used. Overall, one-blastomere biopsy without cryopreservation yielded the highest rate of development to morula (96.1%), expanded blastocyst (84.3%) and hatched blastocyst (74.5%), respectively. When the effect of cryopreservation was taken into account, two-blastomere biopsy did not differ significantly from that of the one-blastomere biopsy in terms of development to the expanded and hatched blastocyst stages. The results of our study suggest that the two-blastomere biopsy with cryopreservation is a better option in the current setup.
Keywords
Embryo biopsy, Cryopreservation, Mouse embryo, Preimplantation genetic diagnosis
Introduction
Preimplantation genetic diagnosis (PGD) involves in vitro manipulations as well as genetic screening. In vitro manipulations include biopsy, embryo culture and if needed, cryopreservation of embryos is opted. Embryos that are subjected to PGD should never be adversely affected by in vitro interventions and must subsequently be able to develop into normal and healthy individuals.
Biopsy of embryos in PGD programs is generally done at the 8-cell stage as it has been reported that biopsy at the 8-cell stage has less detrimental effect on subsequent in vitro development [1], compared with the 4-cell embryos and morulae. Biopsy size has been seen to affect surviv-ability of embryos [2]. Several authors have found that more than 90% of embryos developed into blastocysts after one- and two-blastomere biopsy [2,3,4], but signifi-cant reductions were observed after three to five blas-tomeres were biopsied [2]. Several types of biopsy may be performed, depending on the availability of equipment. Bisection of embryos may be performed using a micro-blade attached to a micromanipulator [5,6,7]. This method has been utilized on livestock embryos before the intro-duction of blastomere biopsy technology in PGD. How-ever, the setback part is the biopsy size which is difficult to control, especially when the biopsy is performed on embryos of smaller animal species.
Currently, blastomere biopsy is the method of choice, as it is more efficient in controlling the biopsy size. Different techniques are adopted in the partial removal or disrup-tion of the zona pellucida before blastomere aspiration is performed. The simplest method uses two microcapillaries for biopsy. The first capillary is used as a holding pipette for the embryo and the other capillary is used to mechanically puncture the zona (zona drilling) followed by blastomere aspiration. This simple method has been adopted in several studies, as it shortens biopsy time [4,8,9]. Other researchers have preferred to partially digest zonae using an acidic medium, such as acidic Ty-rode’s solution [10,11,12]. This type of set-up requires three microcapillary tools. The first tool is used to hold the embryo in place. The second and the third tools are used, respectively to chemically breach the zona with acid and to aspirate blastomeres.
Cryopreservation of biopsied embryos is also required when genetic diagnosis requires a substantial amount of time, or when recipients are not readily available. Cryopreservation methods may be divided broadly into slow freezing and vitrification [13].
Slow freezing involves slow cooling of embryos before storage in liquid nitrogen. This method has been used in several early studies [14,15,16,17].
However, in recent years, vitrification method of cryopreservation has become more popular. This is because vitri-fication protocols are faster and more efficient. Vitrifica-tion involves the use of high concentrations of solutes and rapid cooling rates to avoid intra- and extracellular ice formation. Currently, there are many variations to the vitrification method. Embryo vitrification may be performed using a plastic insemination straw [18,19], a cryoloop made of nylon [20] and microdrops of vitrification solu-tion [21]. This study adopted the vitrification method using plastic insemination straws.
Materials and Methods
Embryo collection
Embryos were collected from 6 to 8-week-old ICR mice. Mice were superovulated by intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (PMSG; Folli-gon, Intervet, Netherlands) followed by 10 IU human chorionic gonadotropin (hCG; Chorulon, Intervet) at 48 h apart. Treated females were then cohabited overnight with a single male of proven fertility, at a ratio of 2:1. The next morning, females with vaginal plugs were selected as embryo donors.
Biopsy
Eight-cell embryos were flushed out of oviducts on day 3 post coitum and each of them were transferred to individual 10-μl drops of calcium-magnesium free M2 (CMF-M2) [22]. One- or two-blastomere biopsies were carried out using the TransferMan® NK 2 (Eppendorf, Germany) fully automated micromanipulator which was mounted on an inverted microscope (Leica DMIRB, Germany) with an in-built heating stage. All microcapillaries were obtained from Eppendorf, Germany. Holding pipettes (VacuTip, Eppendorf) had an inner diameter of 40 μm and outer diameter of 100 μm. Zona drilling tips had an inner diameter of 5 μm and embryo biopsy tips had an inner diameter of 25 μm with a 30-degree angle. A small aperture was then drilled in the zona pellucida using the zona drilling tip containing acidified Tyrode’s solution. Once the hole was drilled, gentle suction was used to draw the blastomere into the tip.
Cryopreservation
Biopsied embryos were cultured for 12 h in M16 medium before being subjected to cryopreservation procedure by vitrification as described by Nagy et al. [23]. Non-biopsied and biopsied embryos were cryopreserved at the same stage of development.
Evaluation of embryo viability
Percentages of embryos developing to morula, blastocyst and hatched blastocyst after biopsy and cryopreservation treatments were recorded as an indicator of embryo vi-ability.
Results
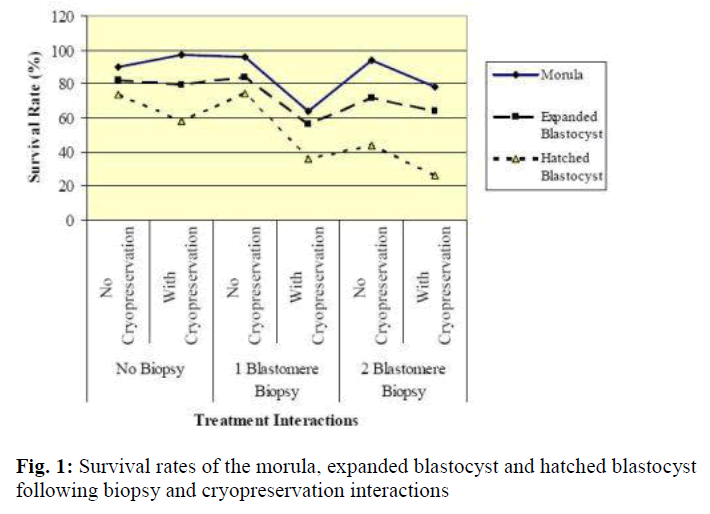
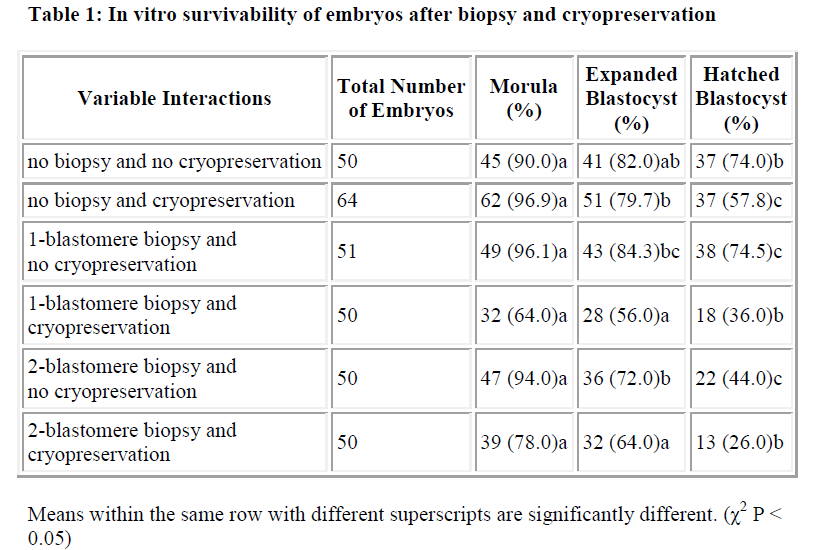
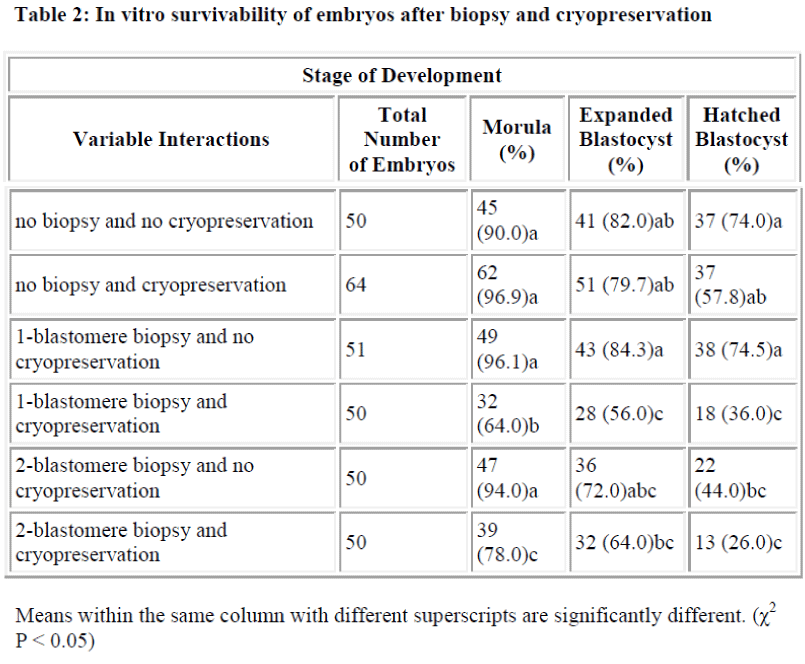
Results of in vitro embryonic survival rates after biopsy and cryopreservation are given in Tables 1 and 2 and also presented in Figure 1.
In vitro survivability of embryos generally declined with the advancing stages of development, irrespective of the treatments (Table 1 and Figure 1).
In comparison with control, the survival rate until morula and expanded blastocyst was not significantly reduced by one-and two-blastomere biopsies without cryopreservation (Table 2). In contrast, the survival rate of the hatched blastocyst was significantly reduced by two-blastomere biopsy compared to the control. No difference was so far detected between one-blastomere biopsy without cryopre-servation and the control.
Following cryopreservation with biopsy interactions (Table 2), the survival rate up to the stage of morula was significantly higher for two-blastomere biopsy with cryopreservation as compared to one-blastomere biopsy with cryopreservation. In disparity the stage of development until the expanded and hatched blastocyst showed no sig-nificant difference between one- and two-blastomere bi-opsies with cryopreservation.
In summary, one-blastomere biopsy without cryopreservation yields the highest development into the morula, expanded blastocyst and hatched blastocyst (96.1%, 84.3% and 74.5%, respectively).
Discussion
It has been demonstrated that the intact and biopsied mouse embryos can successfully be cryopreserved using the blastomere biopsy and vitrification procedures. Both blastocyst formation [1] and hatching rates [25] have been cited as criteria to evaluate the influence of biopsy on the embryo, but the rate of hatching has been suggested as a reliable method to reflect operator’s skills following embryo biopsy [25].
In this study, expanded blastocyst development after two-blastomere biopsy was not found to be significantly dif-ferent from the control. This is in agreement with the finding that biopsy of two blastomeres does not reduce the development of 8-cell embryos to the expanded blastocyst [1]. However, it has been shown that removal of more than three blastomeres from 8-cell embryos significantly impairs the next stage of its development. After biopsy of seven blastomeres, none of the surviving embryos developed into blastocysts [2].
In current PGD practice, the removal of two blastomeres is recommended because of mosaicism [25] and anucleation of blastomeres [26]. In cases of mosaicism, a single cell taken from an embryo may not be an accurate genetic representation of the rest of the embryo [25]. Incidence of anucleation in blastomeres has been reported to be about 2 percent [26]. Therefore, although minimal, there is a risk that a biopsied blastomere may be anucleated. In that case, the genetic testing cannot be carried out.
When cryopreservation effect is taken into account, two-blastomere biopsy did not differ much from that of the one-blastomere biopsy in terms of development to ex-panded and hatched blastocyst (Table 2). Considering the preceding factors, and the advantages of two-blastomere biopsy as mentioned before, the two-blastomere biopsy procedure would be preferred in a PGD set-up.
Cryopreservation is needed when progressive development of the embryo needs to be arrested temporarily. This procedure would be beneficial in cases where the genetic tests take a long time, or when embryo transfer could not be carried out immediately. In the present study, two-blastomere biopsy and cryopreservation interaction produced 78% morulae, 64% expanded blastocyst and 26% hatched blastocyst. Our figures for blastocyst develop-ment showed somewhat lower values than that obtained by other researchers [2]. The authors recorded 80% and 46% of biopsied cryopreserved embryos developed into blastocysts. Another group [27], on the other hand, obtained 46% hatched blastocyst after single blastomeres biopsy and vitrification in straws. This rate is rather closer to the 36% hatched blastocyst that was obtained from one-blastomere biopsy with cryopreservation in the pre-sent study.
However, it is important to note that the experimental data differ as the experimental procedures adopted by the investigators are not absolutely identical. Moreover, in biopsy and cryopreservation experiments, inter laboratory variation also plays a crucial role in determining the out-come.
Acknowledgement
This study was funded by a short-term research grant from the Institute of Research, Development and Commercialisation (IRDC), Universiti Teknologi MARA. Our deepest appreciation to the Dean of the Medical Faculty, Dean of the Faculty of Applied Sciences and Head of the Institute of Medical Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, for their support.
References
- Krzyminska UB, Lutjen J, O’Neill C. Assessment of the viability and pregnancy potential of mouse embryos biopsied at different preimplantation stages of devel- opment. Hum Reprod 1990; 5: 203-208.
- Liu J, van den Abbeel E, van Steirteghem A. The in vitro and in vivo developmental potential of frozen and non-frozen biopsied 8-cell mouse embryos. Hum Reprod 1993; 8: 1481-1486.
- Bodo S, Laczko L, Horvath G, Baranyai B, Horvai Szabo M, Dohy J, Gocza E. A simplified biopsy method for precompacted mouse embryos: A technical report. Acta Vet Hung 2002; 50: 469-479.
- Chen S-U, Ho H-N, Chen H-F, Chao K-H, Huang S-C, Lee T-Y and Yang Y-S. A simplified technique for embryo biopsy: Use of the same micropipette for zona drilling and blastomere aspiration. J Assist Reprod Genet 1997; 14: 157-161.
- Bredbacka P, Kankaanpää A, Peippo J. PCR-sexing of bovine embryos: A simplified protocol. Theriogenology 1995; 44: 167-176.
- Hasler JF, Cardey E, Stokes JE, Bredbacka P. Nonelec-trophoretic PCR-sexing of bovine embryos in a commercial environment. Theriogenology 2002; 58: 1457- 1469.
- Mara L, Pilichi S, Sanna A, Accardo C, Chessa B, Chessa F, Dattena M., Bomboi G, Cappai P. Sexing of in vitro produced ovine embryos (Ovis aries) by duplex polymerase chain reaction. Mol Reprod Dev 2004; 69: 35-42.
- Cui KH, Verma PJ, Matthews CD. Hatching rate: An optimal discriminator for the assessment of single-blastomere biopsy. J Assist Reprod Genet 1993; 10: 157- 162.
- Almodin CG, Moron AF, Kulay Jr. L, Minguietti-Câmara VC, Moraes AC, Torlani MR. A bovine protocol for training professionals in preimplantation genetic diagnosis using polymerase chain reaction. Fertil Steril 2005; 84: 895- 899.
- Gordon JW, Gang I. Use of zona drilling for safe and effective biopsy of mouse oocytes and embryos. Biol Reprod 1990; 42: 869: 876.
- Harper JC, Coonen E, Ramaekers FCS, Delhanty JDA, Handyside AH, Winston RML, Hopman AHN. Identification of the sex of human preimplantation embryos in two hours using an improved spreading method and fluorescent in-situ hybridization (FISH) using directly labelled probes. Hum Reprod 1994; 9: 721- 724.
- Geber S, Sampaio M. Blastomere development after embryo biopsy: A new model to predict embryo development and to select for transfer. Hum Reprod 1999; 14: 782-786.
- Kasai M. Advances in the cryopreservation of mammalian oocytes and embryos: Development of ultra-rapid vitrification. Reprod Med Biol 2002; 1: 1-9.
- Whittingham DG, Leibo SP, Mazur P. Suvival of mouse embryos frozen to -196 degrees and -269 degrees C. Science 1972; 178: 411-414.
- Wilmut I, Rowson, LE. Experiments on the low temperature preservation of cow embryos. Vet Res 1973; 92: 686-690.
- Snabes MC, Cota J, Hughes, MR. Animal investigations: Cryopreserved mouse embryos can successfully survive biopsy and refreezing. J Assist Reprod Genet 1993; 10: 513-516.
- Vitale NJ, Myers MW, Denniston RS, Leibo, SP, God-ke, RA. In-vitro development of refrozen mouse embryos. Hum Reprod 1997; 12: 310-316.
- Kasai M. Cryopreservation of mammalian embryos. Mol Biotechnol 1997; 7: 173-179.
- Nowshari MA, Brem G. Refreezing of mouse intact and biopsied embryos by rapid-freezing procedure. Hum Reprod 2000; 15: 2577-2581.
- Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop containerless technique. Fertil Steril 1999; 72: 1073-1078.
- Dinnyés A, Dai Y, Jiang S, Yang X. High developmental rates of vitrified bovine oocytes following parthenogenetic activation, in vitro fertilization, and somatic cell nuclear transfer. Biol Reprod 2000; 63:513-518.
- Hogan B, Constantini F, Lacy E eds. Manipulating the mouse embryo 1986. Cold Spring Harbor Laboratory Press, New York.
- Nagy A, Gertsenstein M, Vintersten K, Behringer R eds. Manipulating the mouse embryo, A Laboratory Manual Third Edition 2003. Cold Spring Harbor Laboratory Press, New York.
- Cui KH, Verma PJ, Matthews, CD. Hatching rate: An optimal discriminator for the assessment of single-blastomere biopsy. J Assist Reprod Genet 1993;10: 157- 162.
- Harper JC, Delhanty JDA, Handyside AH eds Preimplantation Genetic Diagnosis. 2000 John Wiley & Son, Ltd., United Kingdom.
- Greenlee AR, Krisher RL, Plotka ED.. Rapid sexin of mouse preimplantation embryos using a nested, mltiplex Polymerase Chain Reaction (PCR). Mol Rerod Dev 1998; 49: 261-267.
- Baranyai B, Bodó SZ, Dinnyés A, Gócza, E. Vitrification of biopsied mouse embryos. Acta Vet Hung 205; 53: 103-112.