ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 14
The effects of cardiopulmonary resuscitation under the diaphragmatic muscle on post-resuscitation hemodynamics after cardiac arrest in rabbit models
1Department of Emergency, General Hospital of Chinese People Armed Police Forces, Beijing 100039, PR China
2Intensive Care Unit (ICU), General Hospital of Chinese People Armed Police Forces, Beijing 100039, PR China
3School of Anesthesiology, Xuzhou Medical University, Xuzhou 221002, PR China
- *Corresponding Authors:
- Xiaodong Guo
Intensive Care Unit (ICU)
General Hospital of Chinese People Armed Police Forces, PR China
- Lixiang Wang
Department of Emergency
General Hospital of Chinese People Armed Police Forces, PR China
Accepted date: June 14, 2017
The aim of this study was to compare standard cardiopulmonary resuscitation (S-CPR) and diaphragmatic muscle cardiopulmonary resuscitation (D-CPR) for cardiac arrest (CA) in rabbit models. Twenty rabbits were randomized into either the S-CPR group or the D-CPR group. CA lasting 8 min was induced in healthy New Zealand rabbits through abdominal operation and tracheal tube clamping. During end-expiration 5 min after vital signs stabilized, the aortic pressure and transcutaneous oxygen saturation levels were recorded, and restoration of spontaneous circulation (ROSC) and the 6-h survival rate were analyzed. Five rabbits in the S-CPR group and eight in the D-CPR group achieved ROSC; the survival rate in the S-CPR group and the D-CPR group was 40% and 50%, respectively. Blood pressure indices in each group were higher post-ROSC than at baseline. One minute after resuscitation, the blood pressure values in the D-CPR group were higher than those in the S-CPR group (aortic systolic pressure=54.9 ± 10.1 mmHg versus 42.1 ± 16.2 mmHg, respectively; aortic diastolic pressure=22.1 ± 7.4 mmHg versus 15.1 ± 7.3 mmHg, respectively; mean arterial pressure=33.0 ± 5.8 mmHg versus 21.4 ± 8.5 mmHg, respectively; and coronary perfusion pressure=17.5 ± 7.5 mmHg versus 9.2 ± 6.6 mmHg, respectively). Five minutes after resuscitation, blood pressure values in the D-CPR group remained statistically higher than those in the S-CPR group. However, no differences between the two groups existed after 5 min. When compared to S-CPR, D-CPR resulted in a higher ROSC rate and a higher survival rate in rabbit models; however, the results depend on higher atrial pressure and cardiac output.
Keywords
Cardio-pulmonary resuscitation, Diaphragm, Cardiac compression, Rabbit, Hemodynamics, Survival rate.
Introduction
Cardiac arrest is a major cause of morbidity and mortality worldwide [1], and patients who survive cardiac arrest often develop severe neurological dysfunction due to hypoxic brain injury and reperfusion-induced cell death [2]. Cardiopulmonary resuscitation (CPR) remains the most commonly used resuscitation method for cardiac arrest (CA) [3], and it has been one of the most important determinants of patient survival. However, the quality of CPR, which is related to the depth and frequency of chest compressions [4], always determines the prognosis of CA [5]. On the other hand, standard CPR (S-CPR) can cause many complications including thoracic rib fractures and gastric rupture [6-12]. In addition, although open chest cardiac compression is thought to achieve better outcomes [13], it can also cause major damage and serious postoperative complications.
Additionally, when CA happens during special situations such as open operation, S-CPR disable to act as a thorax pump and fails to ensure the blood supply to major organs because the abdominal cavity is open. Furthermore, the clinical success rate of resuscitation is low (about 10%) and the survival status of patients after resuscitation is not satisfying [14,15]. Therefore, although researchers worldwide have investigated several methods to improve clinical outcomes, they have seen little success [16,17]. Thus, our study aimed to provide theoretical evidence for the clinical applications of diaphragmatic muscle CPR (D-CPR) by comparing the effects of D-CPR and S-CPR on the circulatory function of rabbits before and after resuscitation.
Materials and Methods
Animal preparation
Twenty New Zealand rabbits weighing between 2.0 kg and 3.5 kg (irrespective of the sex) were provided by the animal experimental center of the General Hospital of the People's Liberation Army. The rabbits were randomly assigned into the D-CPR group (n=10) or the S-CPR group (n=10). Ketamine and sumianxin were used to induce anesthesia, the neck trachea was intubated, and the rabbits were connected to a ventilator. A multifunctional physiological monitor was connected to the left carotid artery and the right jugular vein to monitor ascending aortic pressure and right atrial pressure. The electrocardiogram (ECG) results and transcutaneous oxygen saturation (SpO2) levels were monitored during the operation. The ear vein was intubated for transfusion and drug administration. The trachea was clipped at the end of an exhalation to initiate asphyxia. CA was considered successful if the ECG displayed obvious ventricular fibrillation waveforms or a flatline accompanied by a mean arterial pressure (MAP) less than 15 mmHg (1 mmHg=0.133 kPa) and the disappearance of arterial pressure differences or if the monitor displayed an arterial line waveform. We conducted D-CPR or S-CPR exactly 8 min after asphyxia with a massage frequency of 180-200 per min. We simultaneously used a ventilator to assist respiration (frequency: 40 per min, tidal volume: 20 ml/kg, concentration of inspired oxygen: 100%). After 2 min of massage, resuscitation drugs (adrenaline: 0.1 mg/kg, atropine: 0.05 mg/kg) were administered. After 5 min of massage, electric defibrillation was used if there was a ventricular fibrillation waveform on the monitor.
The ventilation mode, respiratory parameters, abdominal incision procedures, and salvage drugs were the same for both cardiac compression methods. For the S-CPR group, the compression depth is that resulting in the thoracic sagged to 1/3-1/2 of its thickness. For the D-CPR group, three or four fingers were inserted into the diaphragm below the heart (the finger pulp was used to place sterile gauze to prevent the fingertips from tearing the diaphragm because of uneven force), and the heart was crimped to the chest wall with a range of 2-3 cm. ROSC was successful when the ECG monitor indicated that the superventricular rhythm was recovered and the MAP was greater than 25 mmHg for more than 1 min.
Hemodynamic determination and treatment
After preparation of the animal models, the rabbits breathed ambient air for 5 min without any operation. After the hemodynamics stabilized, the aortic systolic pressure (AOS), aortic diastolic pressure (AOD), right atrial systolic pressure (RASP), right atrial diastolic pressure (RADP), respiratory rate (RR), heart rate (HR), and SpO2 values were recorded. After asphyxia began, the ascending aortic pressure, right atrial pressure (RAP), ECG results, and SpO2 levels were recorded until the animal achieved ROSC or the experiment was terminated (i.e., if the circulation did not recover after 30 min of massage). The MAP and central venous pressure (CVP, approximately equal to the mean RAP) were calculated. The formulas are as follows: coronary perfusion pressure (CPP)=AOD-RADP; RAP=1/3 × RASP+2/3 × RADP; and MAP=AOD+1/3 × AOS.
Statistical analysis
The statistical software used in this study was SPSS 15.0. The results are displayed using the mean ± standard deviation (x̄ ± s). A t-test was adopted for comparisons among groups, a repeated-measures ANOVA was used for comparing data that changed over time, and a chi-square test (exact probability method) was adopted for categorical data. A P<0.05 was considered statistically significant.
Results
Comparison of the baselines values between the two groups
The baseline values (before CA) for weight, AOS, AOD, MAP, CVP, CPP, HR, RR, and SpO2 were compared between the DCPR group and S-CPR group. No significant differences between the two groups were found (P>0.05 for all values), indicating that the two groups were comparable (Table 1).
| Group | S-CPR | D-CPR | P-value |
|---|---|---|---|
| Number of animals | 10 | 10 | - |
| Weight (kg) | 2.9 ± 0.3 | 2.6 ± 0.4 | 0.107 |
| AOS (mmHg) | 77.7 ± 10.9 | 75.0 ± 4.2 | 0.479 |
| AOD (mmHg) | 57.3 ± 11.4 | 54.0 ± 5.0 | 0.412 |
| MAP (mmHg) | 64.6 ± 10.3 | 60.0 ± 6.5 | 0.294 |
| CVP (mmHg) | 3.7 ± 3.1 | 2.6 ± 2.2 | 0.393 |
| CPP (mmHg) | 55.7 ± 10.8 | 52.4 ± 6.1 | 0.41 |
| HR (times/min) | 173 ± 21 | 171 ± 14 | 0.833 |
| RR (times/min) | 34 ± 7 | 35 ± 5 | 0.831 |
| SpO2 (%) | 97 ± 1.3 | 96 ± 0.6 | 0.104 |
Table 1: The basic value before CA ( ͞x ± s).
Comparison of ROSC between the two groups
Five rabbits in the S-CPR group and 8 rabbits in the D-CPR group achieved ROSC in 5-10 min, but this difference was not statistically significant (P>0.05). One rabbit in the D-CPR group received ventricular fibrillation and achieved ROSC after defibrillation twice; however, resuscitation lasted only 12 min while the survival time for the other rabbits who achieved ROSC was longer than 0.5 h (Table 2).
| Group | Number of animals | Dead (number) | Survive(%(number)) | ROSC rate (%) | ||
|---|---|---|---|---|---|---|
| ≤ 0.5h | ≤ 2.0h | ≤ 6.0h | ||||
| S-CPR | 10 | 5 | 0 (0) | 10 (1) | 40 (4) | 50 |
| D-CPR | 10 | 2 | 10 (1) | 20 (2) | 50 (5) | 80 |
Table 2: The distribution of survival time of animals from D-CPR group and S-CPR group.
Change in hemodynamics 15 min after resuscitation
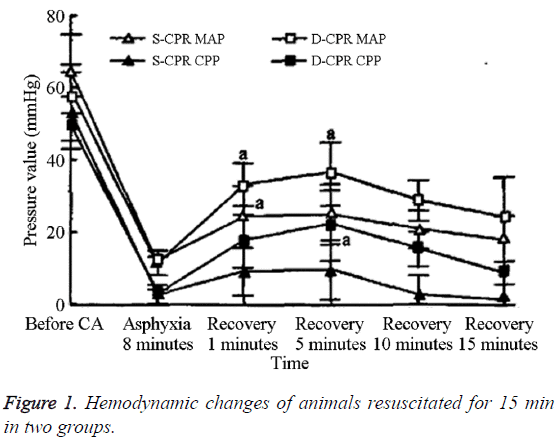
The differences in the frequency of massage and CVP between the two groups at 1 min and 5 min after resuscitation were not statistically significant (Table 3). However, the AOS, AOD, MAP, and CPP values for the D-CPR group were statistically significantly higher than those of the S-CPR group. The MAP and CPP values for the two groups after resuscitation increased significantly until the peak value was reached at 5 min, after which, they decreased gradually (Figure 1). At 1 min and 5 min after resuscitation, the MAP and CPP values for the DCPR group were significantly higher than those of the S-CPR group (P<0.05). However, by 10 and 15 min after resuscitation, the differences between the two groups were no longer statistically significant. Within groups, the AOS, AOD, MAP, and CPP values after CA were significantly lower than the baseline values (Table 4).
| Time after frequency of resuscitation | Group | Animals | Massage (/min) | AOS (mmHg) | AOD (mmHg) | MAP (mmHg) | CVP (mmHg) | CPP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| 1 min | S-CPR | 10 | 188 ± 8 | 42.1 ± 16.2 | 15.1 ± 7.3 | 21.4 ± 8.5 | 16.3 ± 8.5 | 9.2 ± 6.6 |
| D-CPR | 10 | 188 ± 7 | 54.9 ± 10.1* | 22.1 ± 7.4* | 33.0 ± 5.8* | 13.7 ± 3.1 | 17.5 ± 7.5* | |
| 5 min | S-CPR | 10 | 195 ± 17 | 42.6 ± 10.7 | 16.1 ± 9.7 | 24.9 ± 8.4 | 17.9 ± 5.8 | 9.5 ± 8.2 |
| D-CPR | 10 | 186 ± 7 | 57.0 ± 10.0* | 25.5 ± 9.1* | 36.0 ± 8.7* | 13.6 ± 4.1 | 21.9 ± 9.7* |
Table 3: Comparison of the change of hemodynamic in 15 min after resuscitation between two groups (xs).
| Time after resuscitation | Group | Animals | AOS | AOS | AOD | CPP |
|---|---|---|---|---|---|---|
| 1 min | S-CPR | 10 | 52.9% | 26.4% | 37.3% | 16.5% |
| D-CPR | 10 | 73.2% | 40.9% | 54.1% | 33.4% | |
| 5 min | S-CPR | 10 | 55.3% | 28.1% | 38.5% | 17.1% |
| D-CPR | 10 | 76.0% | 47.2% | 60.0% | 41.8% |
Table 4: The ratio of measures and their basic values at different times.
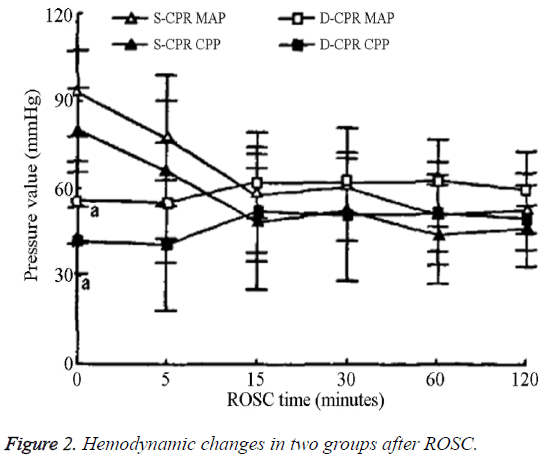
The MAP and CPP values for the S-CPR group were significantly higher than those of the D-CPR group immediately after ROSC; however, these values gradually decreased and became similar to those of the D-CPR group after ROSC (Figure 2). For the D-CPR group, the changes in the MAP and CPP values were steady beginning 5 min post- ROSC and the amount of dopamine used was very small during both the monitoring and maintenance phases.
Complications
Autopsy reports showed that one rabbit in the S-CPR group had pulmonary edema and two had multiple fractured ribs, one of which also had hepatic rupture. One rabbit in the D-CPR group had a diaphragmatic tear at the point of diaphragmatic compression because of the pressure applied directly to the diaphragm by the fingers; however, this situation did not occur after applying gauze at the compression point on the diaphragm.
Discussion
Analysis of the change in hemodynamics
S-CPR is always used for CPR after clinical CA both domestically and abroad. However, experimental studies and clinical practice reveal that hemoperfusion for important organs is not very good for some patients and the success rate of resuscitation is below 30% [18], which might be related to the low perfusion pressure and low blood flow during the period of resuscitation [19-21].
This study revealed that the AOS, AOD, MAP, and CPP values of the D-CPR group were significantly higher than those of the S-CPR group 1 min and 5 min after resuscitation. However, the MAP and CPP values were not significantly different between the two groups 10 min and 15 min after resuscitation. This indicates that D-CPR may result in better hemodynamic effects during the early period of resuscitation (in 5-10 min) and benefit heart hemoperfusion. The decreasing effects of cardiac massage that occurred after more than 5-10 min postresuscitation might be because of decreasing myocardial compliance caused by a long cardiac ischemia and hypoxia duration and a sample size reduction (since the animals continued to achieve ROSC more than 5-10 min postresuscitation). The comparison within groups revealed that the MAP and CPP values 1 min after resuscitation were not significantly different from those 5 min after resuscitation; however, these values were still significantly lower than the baseline values for both groups. In addition, the MAP and CPP values reached 54.1% and 33.4% of the baseline values, respectively, 1 min after resuscitation, and 60.0% and 41.8% of the baseline values, respectively, 5 min after resuscitation. This reveals that D-CPR generates better hemodynamic effects when compared to S-CPR and is in favor of heart hemoperfusion during resuscitation, which might account for the high success rate of resuscitation in the D-CPR group. We also observed that performing CPR while crimping the heart to the chest wall under the diaphragm resulted in good outcomes and few complications for CA patients during liver transplantation and upper abdominal operations (for example, liver, gallbladder, pancreas, or spleen operations) [15-21].
Furthermore, in this study, the rates of ROSC were not significantly different between the D-CPR group and the SCPR group; however, this might be related to the small sample size. Our study also revealed that both the MAP and CPP values decreased after more than 5 min of resuscitation, which might be related to decreasing myocardial compliance due to a long cardiac ischemia and hypoxia duration.
The D-CPR mechanism
Our study revealed that the AOS, AOD, MAP, and CPP values of the D-CPR group were significantly higher than those in the S-CPR group during resuscitation. This verifies that cardiac compression through the diaphragm could partially construct effective artificial circulatory support to maintain blood circulation and ensure hemoperfusion for important organs. The D-CPR can generate artificial circulation and ventilation via the thoracic, abdominal and heart pump mechanisms. DCPR results in upward and downward motion of diaphragm and pressure changes in the abdominal cavity, which actives the “abdominal pump”. The piston effect of the diaphragm in the thoracic and abdominal cavities then transmits pressure changes in the abdominal cavity to the thoracic cavity, inducing thoracic pressure changes which indirectly activate the “thoracic pump”. The anatomical relationship of the heart and the diaphragm then activates the “heart pump, which results in blood flow. In addition, adequate coronary perfusion pressure (CPP) is important for successful CPR, and abdominal compression can significantly increase CPP. An advantage of abdominal pumping on the chest is that it would promote some ventilation. So rhythmic diaphragm compression CPR provides effective blood circulation and some oxygen supplement. It may be the reason why D-CPR has better hemodynamic effects when compared to S-CPR.
In conclusion, the evidence provided in this study indicates that D-CPR results in better outcomes when compared to S-CPR. These results provide additional experimental supporting the popularization of D-CPR in clinical practice. However, the situation in clinical practice is complicated and variable, and animal models cannot completely mimic a real clinical situation. Therefore, additional, large-scale experiments are needed to verify the results of our experiment.
References
- Patel JK, Chabra V, Parnia S. Making sense of clinical outcomes following cardiac arrest. Curr Opin Crit Care 2015; 21: 453-459.
- Rawal G, Yadav S, Garg N. Therapeutic Hypothermia after Prolonged Cardiac Arrest: Case Report with Review of Literature. J Clin Diagn Res 2015; 9: OD01-2.
- Reynolds JC, Bond MC, Shaikh S. Cardiopulmonary resuscitation update. Emerg Med Clin North Am 2012; 30: 35-49.
- Wang J, Tang C, Zhang L, Gong Y, Yin C, Li Y. Compressing with dominant hand improves quality of manual chest compressions for rescuers who performed suboptimal CPR in manikins. Am J Emerg Med 2015; 33: 931-936.
- Yannopoulos D, Aufderheide TP, Abella BS, Duval S, Frascone RJ, Goodloe JM, Mahoney BD, Nadkarni VM, Halperin HR, O'Connor R, Idris AH, Becker LB, Pepe PE. Quality of CPR: An important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation 2015; 94: 106-113.
- Derwall M, Brücken A, Bleilevens C, Ebeling A, Föhr P, Rossaint R, Kern KB, Nix C, Fries M. Doubling survival and improving clinical outcomes using a left ventricular assist device instead of chest compressions for resuscitation after prolonged cardiac arrest: a large animal study. Crit Care 2015; 19: 123.
- Aufderheide TP, Pirrallo RG, Provo TA, Lurie KG. Clinical evaluation of an inspiratory impedance threshold device during standard cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest. Crit Care Med 2005; 33: 734-740.
- Reiger J, Eritscher C, Laubreiter K, Trattnig J, Sterz F, Grimm G. Gastric rupture--an uncommon complication after successful cardiopulmonary resuscitation: report of two cases. Resuscitation 1997; 35: 175-178.
- Aufhauser DD Jr, Rose T, Levine M, Barnett R, Ochroch EA, Aukburg S, Greenblatt E, Olthoff K, Shaked A, Abt P. Cardiac arrest associated with reperfusion of the liver during transplantation: incidence and proposal for a management algorithm. Clin Transplant 2013; 27: 185-192.
- Hoke RS, Chamberlain D. Skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 2004; 63: 327-338.
- Lederer W, Mair D, Rabl W, Baubin M. Frequency of rib and sternum fractures associated with out of hospital cardiopulmonary resuscitation is underestimated by conventional chest X ray. Resuscitation 2004; 60: 157-162.
- Kim MJ, Park YS, Kim SW, Yoon YS, Lee KR, Lim TH, Lim H, Park HY, Park JM, Chung SP. Chest injury following cardiopulmonary resuscitation: a prospective computed tomography evaluation. Resuscitation 2013; 84: 361-364.
- Benson DM, O'Neil B, Kakish E, Erpelding J, Alousi S, Mason R, Piper D, Rafols J. Open-chest CPR improves survival and neurologic outcome following cardiac arrest. Resuscitation 2005; 64: 209-217.
- Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, Lamb SE, Slowther AM, Woollard M, Carson A, Smyth M, Whitfield R, Williams A, Pocock H, Black JJ, Wright J, Han K, Gates S. PARAMEDIC trial collaborators. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015; 385: 947-955.
- Gianotto-Oliveira R, Gianotto-Oliveira G, Gonzalez MM, Quilici AP, Andrade FP, Vianna CB, Timerman S. Quality of continuous chest compressions performed for one or two minutes. Clinics (Sao Paulo) 2015; 70: 190-195.
- Nozari A, Rubertsson S, Gedeborg R, Nordgren A, Wiklund L. Maximisation of cerebral blood flow during experimental cardiopulmonary resuscitation does not ameliorate post--resuscitation hypoperfusion. Resuscitation 1999; 40: 27-35.
- Smith T. Alternative cardiopulmonary resuscitation devices. Curr Opin Crit Care 2002; 8: 219-223.
- Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O'Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122: S640-656.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112: IV1-203.
- Safar P, Behringer W, Böttiger BW, Sterz F. Cerebral resuscitation potentials for cardiac arrest. Crit Care Med 2002; 30: S140-144.
- Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, Paradis NA. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med 2010; 28: 195-202.