ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 13
The extract of Lycium barbarum L. improves the behavior of rats with premature ovarian by regulating related genes
Department of Traditional Medicine, the Seventh People's Hospital of Shanghai, the Pudong District of Shanghai, PR China
- *Corresponding Author:
- Yumei Ye
Department of Traditional Medicine
The Seventh People's Hospital of Shanghai
The Pudong District of Shanghai, PR China
Accepted date: May 22, 2017
Objective: To explore the effect of Lycium barbarum L. on premature ovarian and the related mechanism.
Methods: The ingredients of Lycium barbarum L. were extracted with water and alcohol, respectively. Rats with premature ovarian were treated with water extractive and ethanol extractive, respectively. After treatment, the biological behaviors, weight of ovary and uterus of rats were determined and compared. The contents of E2, FSH, and LH were determined by ELISA. The protein expression values of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 were determined by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western blotting, respectively.
Results: Both water extractive and ethanol extractive could improve the behaviors of rats. Water extractive could obviously increase the content of estrogen in blood and the size of uterine. Compared with ethanol extractive, water extractive could obviously regulate the expression of premature ovarian related genes.
Conclusion: Both water extractive and ethanol extractive have roles in treating rats with premature ovarian. Moreover, the treatment result of water extractive was above the ethanol extractive.
Keywords
Lycium barbarum L., Premature ovarian, Water extractive, Ethanol extractive, Biological behavior.
Introduction
Premature Ovarian Failure (POF) is the loss of ovarian function which is characterized by amenorrhea, hypoestrogenism, and elevated serum gonadotropin levels in women younger than the age of 40 [1]. The estimated incidence of POF is 1:10000 women by age 20; 1:1000 women by age 30; and 1:100 women by age 40 [2]. Women with POF experience menopausal symptoms such as hot flashes, vaginal dryness, dyspareunia, insomnia, vaginitis, and mood swings [3].
For thousands of years, Chinese Herbal Medicine (CHM) has been used to treat many diseases. Traditional Chinese Herbal Medicine (TCHM) contains multiple botanicals, each of which contains many compounds that may be relevant to the medicine's putative activity [4]. The philosophy of Chinese Medicine in terms of biology, pathophysiology and pharmacology takes an entirely different approach. Up to now, the diagnostic and therapeutic modalities rely on experience and social customs, and many treatments still do not have a concise mechanism of action and clear side effect profile [5]. Currently, CHM are mainly used to decrease the risk and treat common pathologies i.e. hypertension, hypercholesterolemia, diabetes mellitus, obesity, cancer, depression, prevention of miscarriages and even POF. Animal experiments have demonstrated the efficacy of Chinese herbs to inhibit the production of Anti-ovarian Antibodies (AoAb) and to further protect the ovaries from autoimmune destruction [6]. Studies have been done to reveal CHM’s influence on the serum level of hormones and its involvement with the Hypothalamic- Pituitary-Ovarian (HPO) axis [7]. CHM is also effective in the management of the infertility caused by POF [8].
Lycium barbarum Linnaeus, a well-known Chinese herb which is widely consumed by oriental people, has been shown to exhibit anti-cancer [9]. The fruit of Lycium barbarum Linnaeus contain functional components such as carotenoids, flavonoids and polysaccharides [10]. Lycium barbarum L. has been regarded as an upper class Chinese medicine in Chinese pharmacopoeia, indicating that the fruit of L. barbarum can be one of the ingredients in Chinese cuisine or formulated Chinese medicine [11].
In this study, we aimed to explore the treatment of water and ethanol extractive from Lycium barbarum L. on POF. The effect of water and ethanol extractive from Lycium barbarum L. on POF was also compared.
Materials and Methods
Animals
Female Specific Pathogen-Free (SPF)-grade Sprague-Dawley (SD) rats, weighing 200-220 g, as well as the standard basal diet, were supplied by Guangdong Medical Laboratory Animal Center. The rats were acclimatized to the facilities for 3 d, and the estrum cycle was observed every day. Rats with two continuous estrum were chosen for experiments and randomly divided into five groups (n=30): control, model, low Lycium barbarum L. (1 mg/g body weight), middle Lycium barbarum L. (10 mg/g body weight) and high Lycium barbarum L. (100 mg/g body weight).
Acousto-optical-electric stimulation (acousto-optical for 10 s, acousto-optical- electric for 60 s, and electrical stimulation for 5 s) was applied to prepare the model for 20 d, five times per day, at random intervals. Each process was completed within 1 h. Acousto, optical, and electrical stimuli used the following constant parameters: 1) sound intensity of 65 dB; 2) illumination intensity of 500 lux with shining and frequency of 1/s, 500 lux with lights on, and 300 lux with lights off; and 3) voltage of 24-36 V.
Plant materials
The medical material of Lycium barbarum L. was purchased from Medical Materials Company of Guangzhou City.
Drugs were administered at the same day post-modeling. Distilled water was given to rats in the control and model group, while drugs were given to corresponding experimental groups once per day for 25 d (drugs were given another continuous 5 d after stress stimulation for 20 d). Biological behaviours of rats in the five groups were observed. The weights of ovary and uterus were also determined.
Enzyme-linked immunosorbent assay
The protein expression levels of E2, FSH and LH in serum were determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum of 100 g after frozen in liquid nitrogen or cell lines was put into homogenizer and 3 times the volume of normal saline was added for ice bath homogenate. The homogenate was centrifuged at 2000 rpm, 4°C for 20 min and the supernatant was collected. After incubated for 1 h at 37°C, the mixture were added with 100 μL streptavidin-Horse Radish Peroxidase (HRP) and incubated for another 30 min. Then, 100 μL stabilized chromogen was put with 100 μL stop buffer and the adsorption value of mixture at 450 nm was read and recorded.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cell lines using TRIzol method. Trizol of 1 ml was added and the solution was mixed homogeneously for 10 min. The mixture was then transferred into eppendorf tubes (EP, 1.5 ml) with 200 μL chloroform. After 15 min shaking, the EP tubes were centrifuged at 4°C for 15 min (12000X g). The supernate was transferred into other EP tubes and mixed with isopyknic isopropanol for 15 s. The centrifugation (4°C, 10 min, 12000 Xg) was carried out again and the supernate was discarded. The precipitate was washed by 75% ethonal twice and dissolved into 30 μL Diethypyrocarbonate (DEPC) after dried to obtain RNA stock solution. After isolation, the concentration of RNA was assessed using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware, USA) and the RAN solution was stored at -80°C for further use. Genes were amplified by specific oligonucleotide primer, and human Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene was used as an endogenous control. The detection and quantification contained the following steps: first, reverse transcription was performed at 55°C for 30 min, initial activation for 15 min at 95°C, next 40 cycles of denaturation were conducted at 94°C for 15 s, then annealing for 30 s at 55°C, extension for 30 s at 72°C. The expression level was normalized using U6 small nuclear RNA by the 2-ΔCt method. The ΔCt values were normalized to GAPDH level. The sequence of primers are as follows: PLA2G4A: GTGCGACAGGAACCAGAAGC and CTCGGGCTTCTGGTTCCTGT, PAPPA: GGCTGTCAGAAGGCCAGATG and TGCCATGAAGGGATTTGAAGA, STC2: AATGGATCATCTCCACTATCACC and CTTACATGGGATTTGCATGACTT, CCL2: CAGCCAGATGCAATCAATGCC and TGGAATCCTGAACCCACTTCT, NELL1: GCTTTGGGATGGACCCTGAC and GAAATAAAAATGCTTTGCTGGC and GAPDH: GAGAGACCCTCACTGCTG and GATGGTACATGACAAGGTGC.
Western blot assay
Cells were washed twice with ice-cold PBS and then lysed in RIPA buffer with protease and phosphatase inhibitors (Beyotime Biotechnology, China). Protein concentrations were determined using a BCA protein assay kit (KeyGen Biotech). Equivalent amounts of total proteins (40 μg) were loaded onto a 10% SDS-polyacrylamide gel and then transferred onto polyvinylidine difluoride membranes. The transferred membranes were blocked for 1 h in 5% nonfat milk and then incubated with the primary antibodies for MGC803, AGS, BGC823 and SGC7901 (Santa Cruz Biotechnology, SantaCruz, CA, USA) at 4°C overnight. Membranes were washed three times with TBSH for 10 min and incubated with horseradish peroxidase-coupled secondary antibodies for 2 h at room temperature. The immunoreactive bands were visualized using enhanced chemiluminescence with ECL reagents (Pierce, Rockford, IL). Each assay was repeated three times and β-actin protein level was used as a control for equal protein loading.
Statistical analysis
Data were processed using SPSS 19.0 statistical software (SPSS, Inc., Chicago, IL, USA) and recorded as the mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference. In addition, one-way analysis of variance was adopted to assess the data. All of the experiments were performed in triplicate for the purposes of comparison.
Results
Biological behavior changes
Rats in the POF model group had stiff and dull hair, exhibited behaviors of chasing and biting other rats, and did not undergo weight gain during the study period. In contrast, POF model rats treated with medium- and high-dose water and ethanol extractive from Lycium barbarum L. had neat and supple hair, exhibited more docile behaviors, and experienced the normal increase in weight during the study period. POF model rats treated with low-dose water and ethanol extractive from Lycium barbarum L. displayed characteristics and behaviors that were similar to those in the untreated POF model group, indicating that the low dose had no evident effect on POF symptoms.
Ovarian and uterine weight changes
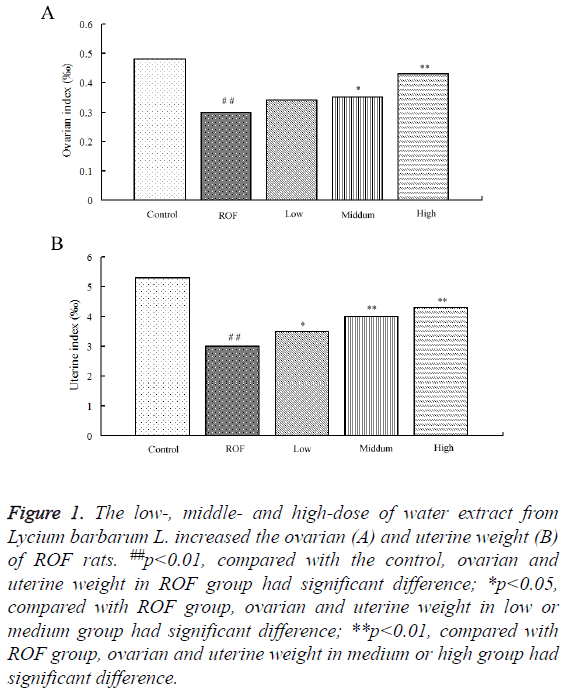
To study the effect of water extractive from Lycium barbarum L. on ROF models, the ovarian and uterine weight in ROF rats after treated with low-, middle- and high-dose of water extractive were compared. Results showed the ovarian weight of ROF model rats decreased significantly compared with the control rats. The ovarian weight of ROF rats increased after treated with low-, middle- and high-dose of water extractive. The additional proportion increased with the increase of water extractive concentration (Figure 1A). The study on uterine weight showed the uterine weight of ROF rats increased after treated with low-, middle- and high-dose of water extractive. Moreover, the uterine weight of ROF rats after treated with high-dose of water extractive was significantly higher than that in ROF models and almost the same with that in the control (Figure 1B).
Figure 1: The low-, middle- and high-dose of water extract from Lycium barbarum L. increased the ovarian (A) and uterine weight (B) of ROF rats. ##p<0.01, compared with the control, ovarian and uterine weight in ROF group had significant difference; *p<0.05, compared with ROF group, ovarian and uterine weight in low or medium group had significant difference; **p<0.01, compared with ROF group, ovarian and uterine weight in medium or high group had significant difference.
The content of E2, FSH, and LH changes
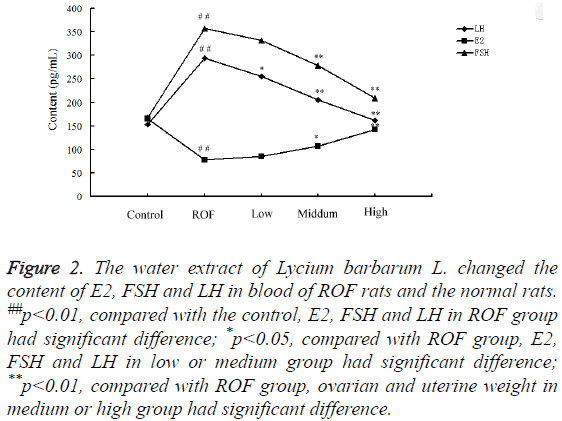
The contents of E2, FSH, and LH in blood of rats in the control, ROF, low, medium and high groups were determined by ELISA. Results showed the contents of LH and FSH were enhanced markedly in ROF rats compared with the control. After treating with low-, middle- and high-dose of water extractive from Lycium barbarum L., the content decreased gradually and was almost the same with that in the control when treated with high-dose of water extractive (Figure 2). The content of E2 was significantly reduced in ROF model rats compared with the control. The value changed after treating with water extractive and increased with the increase of water extractive content.
Figure 2: The water extract of Lycium barbarum L. changed the content of E2, FSH and LH in blood of ROF rats and the normal rats. ##p<0.01, compared with the control, E2, FSH and LH in ROF group had significant difference; *p<0.05, compared with ROF group, E2, FSH and LH in low or medium group had significant difference; **p<0.01, compared with ROF group, ovarian and uterine weight in medium or high group had significant difference.
The content of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 changes
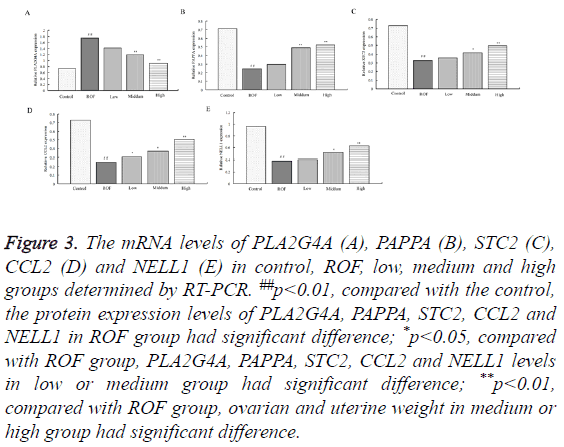
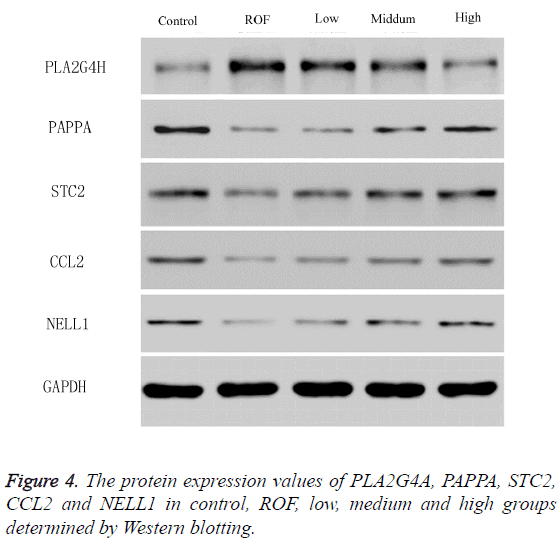
To study the influence of water extractive from Lycium barbarum L. on the levels of PLA2G4A, PAPPA, STC2, CCL2 and NELL1, RT-PCR and western blotting were used. The mRNA levels of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 determined by RT-PCR were shown in Figure 3. As shown, the mRNA level of PLA2G4A in ROF model rats was significantly enhanced compared with those in the control. The level decreased gradually with the increase of water extractive concentration. The level was almost the same with that in the control after treating with high dose of water extractive (Figure 3A). The levels of PAPPA, STC2, CCL2 and NELL1 were reduced in ROF model rats compared with the control. After ROF rats were treated with water extractive from Lycium barbarum L., the levels increased. The values increased gradually with the increase of water extractive concentration and were almost the same with the control when treating with high dose of water extractive (Figures 3B-3E). To further explore the influence, the protein expression values of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 were determined by Western blotting. Results of Western blotting stayed the same with those in RT-PCR (Figure 4).
Figure 3: The mRNA levels of PLA2G4A (A), PAPPA (B), STC2 (C), CCL2 (D) and NELL1 (E) in control, ROF, low, medium and high groups determined by RT-PCR. ##p<0.01, compared with the control, the protein expression levels of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 in ROF group had significant difference; *p<0.05, compared with ROF group, PLA2G4A, PAPPA, STC2, CCL2 and NELL1 levels in low or medium group had significant difference; **p<0.01, compared with ROF group, ovarian and uterine weight in medium or high group had significant difference.
Discussion
Premature Ovarian Failure (POF) is a primary ovarian defect characterized by absent menarche (primary amenorrhea) or premature depletion of ovarian follicles before the age of 40 years (secondary amenorrhea) [12]. It causes hypergonadotrophic hypogonadism occurs in 1% of women. In majority of cases the underlying cause is not identified. The known causes include: genetic aberrations, autoimmune ovarian damage, iatrogenic following surgical, radiotherapeutic or chemotherapeutic interventions as in malignancies and environmental factors like viral infections and toxins [13]. Chinese Herbal Medicines (CHM) is increasingly being used as an alternative treatment for many diseases. It is a common complementary therapy used by patients with cancer for reduction of chemotherapy-induced toxic effects [14]. Fruit from Lycium barbarum L. in the family Solanaceae is wellknown in traditional Chinese herbal medicine and nowadays has been widely used as a popular functional food, with a large variety of beneficial effects, such as reducing blood glucose and serum lipids, anti-aging [15-17]. Lycium barbarum fruits can be used to produce various types of healthy products and foods, e.g., medicinal beverages and drinks, and healthy dietary soups [18]. Some constituents of Lycium barbarum fruits have been chemically investigated, especially Lycium barbarum polysaccharide (LBP) components. Five polysaccharides (glycoconjugates) (LbGp1-LbGp5) were isolated and structurally elucidated [19]. Lycium barbarum polysaccharide had effects on antitumor activity and immune function [20].
In this study, we extracted the ingredients from Lycium barbarum L. by water and a certain concentration of ethanol (50%). The water and ethanol extractive were used to treat ROF rats prepared previously and the biological behaviour of rats were observed. Results showed both water extractive and ethanol extractive improved the behaviors of rats. However, water extractive improved the estrogen content and uterine size more significantly than ethanol extractive. Therefore, low-, middle- and high-dose of water extractive were used for further study. Research showed water extractive could enhance ovarian and uterine weight, and the values increased with the increase of water extractive concentration. Therefore, we speculated the water extractive of lycium barbarum L. had some therapeutic effect on ROF. Then, we studied the molecular mechanism about the treatment of Lycium barbarum L. on ROF.
The levels of ROF related gene E2, FSH, and LH in serum of rats in the control, ROF, low, medium and high groups were determined. Results showed high concentration of water extractive decreased the level of FSH and LH significantly. Follicle stimulating hormone (FSH) is a member of the glycoprotein hormone family that includes luteinizing hormone (LH), thyroid stimulating hormone, and chorionic gonadotropin [21]. Gonadal function is controlled by the two pituitary gonadotropins, LH and FSH [19]. While LH mainly regulates gonadal steroidogenesis, FSH is considered essential for folliculogenesis in the female and spermatogenesis in male. It stimulates in ovarian granulosa cells diverse, differentiationdependent responses that implicate activation of specific cellular signaling cascades [22]. Pituitary gonadotropins FSH and luteinizing hormone stimulate the gonads by regulating germ cell proliferation and differentiation [23]. The content of E2 in ROF model rats was markedly lower than that in the control. After treating with water extractive from Lycium barbarum L., the content was significantly increased. Afterwards, we determined the mRNA levels and protein expression values of PLA2G4A, PAPPA, STC2, CCL2 and NELL1 in ovary by RT-PCR and Western blotting. Results showed water extractive from Lycium barbarum L. increased the levels of PAPPA, STC2, CCL2 and NELL1, and decreased the level of PLA2G4A. Two polymorphisms of the PLA2G4A have been found in chromosome 1q25-the polyA polymorphism and the BanI polymorphism, which occur near the promoter region and near the first intron, respectively [24]. The human glycoprotein, stanniocalcin 2 (STC2) plays multiple roles in several tumor types [25]. STC-2 contains a cluster of histidine residues in the C-terminal portion of the protein. It was previously shown to decrease phosphate uptake into kidney cells in vitro [26]. It is previously known to be involved in the regulation of calcium and phosphate transport in the kidney and also reported to play multiple roles in several cancers [27]. CCL2 plays a key role in leukocyte migration and are potential targets in the treatment of chronic inflammatory disorders [28]. It has been demonstrated to recruit monocytes to tumor sites [29]. It promotes prostate cancer tumorigenesis and metastasis [30]. NELL1 is an extracellular protein inducing osteogenic differentiation and bone formation of osteoblastic cells [31]. It encodes cysteine-rich amino acid sequences including six epidermal growth factor-like motifs, which contain signal peptides at the N-terminals [32]. Therefore, we speculated water extractive from Lycium barbarum L. improved ROF by regulating ROF related gene FSH, E2, LH, PLA2G4A, PAPPA, STC2, CCL2 and NELL1.
In conclusion, Lycium barbarum L. has effect on treating ROF by regulating the expression of related gene FSH, E2, LH, PLA2G4A, PAPPA, STC2, CCL2 and NELL1.
Acknowledgement
The study was supported by fund of Pudong new area of TCM heir PDZYXK-6-2014025.
References
- Baker VL. Primary ovarian insufficiency in the adolescent. Current Opinion in Obstetrics Gynecol 2013; 25: 375-381.
- Coulam CB, Adamson SC Annegers JF. Incidence of premature ovarian failure. Obstetrics Gynecol 1986; 67: 604-606.
- Zhou K. Electroacupuncture modulates reproductive hormone levels in patients with primary ovarian insufficiency: results from a prospective observational study. Evidence Based Compl Alt Med 2013.
- Xie P. Chromatographic fingerprint analysis-a rational approach for quality assessment of traditional Chinese herbal medicine. J chromatogr A 2006; 1112: 171-180 .
- Guo Z. Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Analytica Chimica Acta 2001; 436: 41-47.
- Zhu L, Luo S, Xu L. Influence of Zuogui pill on antiovarian antibodies in immune premature ovarian failure rats. Mod J Integr Chinese Trad West Med 2006; 15: 435.
- Liu HP. Effect of bushenhuoxue prescription on Bcl-2/Bax protein of apoptosis contress genes in follicle of immune premature ovarian failure mice. Chinese Arch Trad Chinese Med 2010; 5: 58.
- Chao SL, Huang LW, Yen HR. Pregnancy in premature ovarian failure after therapy using Chinese herbal medicine. Chang Gung Med J 2003; 26: 449-452.
- Lin C. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int J Biol Macromol 2009; 45: 146-151.
- Inbaraj BS. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J Pharm Biomed Anal 2008; 47: 812-818.
- Chang RCC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol 2008; 28: 643-652.
- Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis 2006; 1: 1.
- Goswami D, Conway GS. Premature ovarian failure. Human Reprod Update 2005; 11: 391-410.
- Mok T. A double-blind placebo-controlled randomized study of Chinese herbal medicine as complementary therapy for reduction of chemotherapy-induced toxicity. Ann Oncol 2007.
- Hu X. Effect of Gosha-jinki-gan (Chinese herbal medicine: Niu-Che-Sen-Qi-Wan) on insulin resistance in streptozotocin-induced diabetic rats. Diab Res Clin Pract 2003; 59: 103-111.
- Chen XJ, Oppenheim, Howard OZ. Shikonin: a component of antiinflammatory Chinese herbal medicine, selectively blocks chemokine binding to CC chemokine receptor-1. Int Immunopharmacol 2001; 1: 229-236.
- Luo Q. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci 2004; 76: 137-149.
- Li Q. Healthy functions and medicinal prescriptions of Lycium barbarum (Gou Ji Zi). Jindun Press Beijing 2001.
- Peng XM. Studies on chemistry and immun‐modulating mechanism of a glycoconjugate from Lycium barbarum L. Chinese J Chem 2001; 19: 1190-1197.
- Gan L. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur J Pharmacol 2003; 471: 217-222.
- Kumar TR. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature Gene 1997; 15: 201-204.
- Gonzalez-Robayna IJ. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 2000; 14: 1283-1300.
- Dierich A. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Nat Acad Sci 1998; 95: 13612-13617.
- Pae CU. BanI polymorphism of the cytosolic phospholipase A2 gene may confer susceptibility to the development of schizophrenia. Progress in Neuro-Psychopharmacology Biol Psych 2004; 28: 739-741.
- Wang H. STC2 is upregulated in hepatocellular carcinoma and promotes cell proliferation and migration in vitro. BMB Rep 2012; 45: 629-634.
- White KE. Chromosomal localization of two human genes involved in phosphate homeostasis: the type IIb sodium-phosphate cotransporter and stanniocalcin-2. Somatic Cell Mol Gene 1998; 24: 357-362.
- Na SS. Stanniocalcin-2 (STC2): a potential lung cancer biomarker promotes lung cancer metastasis and progression. Biochimica et Biophysica Acta (BBA) Proteins Proteom 2015; 1854: 668-676.
- Haringman JJ. A randomized controlled trial with an anti‐CCL2 (anti–monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheumat 2006; 54: 2387-2392.
- Mizutani K. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia 2009; 11: 1235-1242.
- Zhang JLP, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 2010; 21: 41-48.
- Bokui N. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett 2008; 582: 365-371.
- Maeda K. Brain specific human genes, NELL1 and NELL2, are predominantly expressed in neuroblastoma and other embryonal neuroepithelial tumors. Neurologia Medico-Chirurgica 2001; 41: 582-589.