ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 17
The relation between plasma levels of thrombomodulin and endothelial dysfunction in Syrian children with acute lymphoblastic leukemia
Rana Aldabbas* and Jumana Alsaleh
Department of Biochemistry and Microbiology, Faculty of Pharmacy, Damascus University, Damascus, Syria
- *Corresponding Author:
- Rana Aldabbas
Department of Biochemistry and Microbiology
Faculty of Pharmacy
Damascus University, Syria
Accepted date: May 29, 2017
Background: Acute Lymphoblastic Leukemia (ALL) is the most common childhood malignancies, representing nearly one-third of all pediatric cancers. Thrombomodulin is a membrane glycoprotein expressed on endothelial cells. Its plasma level depends on the integrity of the endothelium. Soluble thrombomodulin is derived from injured endothelial cells or proteolytically cleaved from thrombomodulin by proteases. In the past, the endothelium was considered to be inert, described as a 'layer of nucleated cellophane', with only non-reactive barrier properties. However, it is now becoming clear that endothelial cells actively and reactively participate in hemostasis and immune and inflammatory reactions. They regulate vascular tone via production of nitric oxide, endothelin and prostaglandins. Severe endothelial dysfunction is present during the acute phase of acute lymphoblastic leukemia and it result from the disease itself, from treatment, or from other conditions (e.g. sepsis).
Objective: The aim of this study was to determine the level of serum soluble thrombomodulin as a marker of endothelial activation in children with ALL at time of diagnosis and after the chemotherapy.
Methods and Materials: A case-control study included thirty patients with ALL and twenty healthy children. We analysed serum soluble thrombomodulin levels by enzyme-linked immunosorbent assay.
Results: In children with acute lymphoblastic leukemia, there was a significant increase in soluble thrombomodulin levels during the acute phase of the disease and during treatment.
Conclusion: Severe endothelial dysfunction is present during the acute phase of ALL and during treatment and appears to result from the disease itself and from the treatment.
Keywords
Acute lymphoblastic leukemia (ALL), Thrombomodulin, Endothelial dysfunction
Introduction
Acute lymphoblastic leukemia is one of the most common childhood malignancies [1], representing nearly one-third of all pediatric cancers with a peak incidence in children aged 2-5 y [2].
Thrombomodulin (TM) is a cell surface-expressed transmembrane glycoprotein originally identified on vascular endothelium [3]. TM is a glycosylated type I transmembrane molecule of 557 amino acids with multiple domains. Each domain possesses distinct properties. The molecule consists of an NH2-terminal lectin-like region followed by six tandem Epidermal Growth Factor (EGF)-like structures, an Oglycosylation site-rich domain, a transmembrane domain, and a cytoplasmic tail domain. It is a cofactor for thrombin binding that mediates protein C activation and acts as an anticoagulant factor. In addition to its anticoagulant activity TM also possesses antifibrinolytic, cytoprotective and antiinflammatory activities [4-7]. TM is present in the body in two forms with different molecular weights. The first type has a higher molecular weight, and it is bound to cytoplasmic membrane of endothelial cells; the second form has a lower molecular weight and represents the soluble or plasmatic form. The soluble Thrombomodulin (sTM) is derived from injured endothelial cells or proteolytically cleaved by neutrophil elastase and by other substances produced during acute and chronic inflammatory responses, immunologic reactions and complement activation [8-10].
Severe endothelial dysfunction is present during the acute phase of acute lymphoblastic leukemia This dysfunction can result from the disease itself, from treatment, or from other conditions, e.g. sepsis [11,12].
Materials and Methods
We studied 30 patients (17 girls and 13 boys) newly diagnosed with ALL (26 B cell and 4 T cell) aged 1-13 y and a control group which consisted of 20 healthy children matched for age and sex.
Samples were collected at time of diagnosis and on day 35 after induction phase of chemotherapy which includes Glucocorticoids (GC), L-Asparaginase (l-ASP), Vincristen (VCR) and anthracyclines (Danurobicine).
Two milliliters of venous blood were obtained from patients. Samples were collected into tubes preloaded with sodium citrate and then Platelet-poor citrated plasma was obtained from venous blood by centrifugation at 3000 revolutions per min for 10 min. Plasma was then aliquoted and frozen at -70°C for subsequent batch analysis.
Plasma levels of TM were measured using commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kit according to the manufacturer's instructions.
Statistical analysis
Data were analysed using SPSS (version 20, IBM SPSS). Results were presented as mean ± SD (Standard Deviation). Comparison between the mean of two independent groups was performed using Mann-Whitney' U-test and P<0.05 was considered statistically significant.
Results
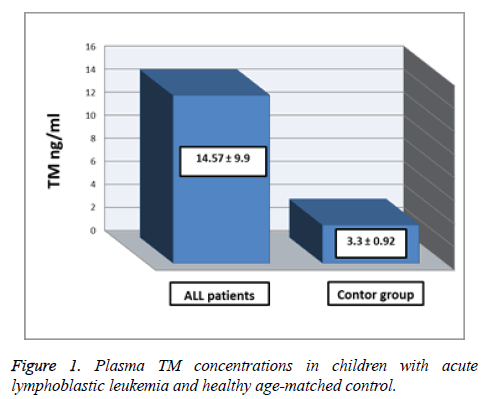
The Table 1 and Figure 1 show the comparison between plasma TM concentrations in children with acute lymphoblastic leukemia at time of diagnosis and healthy agematched controls. The mean of plasma TM concentrations was significantly higher in Acute Lymphoblastic Leukemia (ALL) patients (14.57 ± 9.9 ng/ml) than control group (3.33 ± 0.92 ng/ml), (p<0.05).
| Group | Mean ± SD | Number | Minimum | Maximum |
|---|---|---|---|---|
| ALL patients at time of diagnosis | 14.57 ± 9.9 ng/ml | 30 | 5.02 | 35.55 |
| Control | 3.33 ± 0.92 ng/ml | 20 | 2.09 | 5.23 |
Table 1. Plasma TM concentrations in children with acute lymphoblastic leukemia (at time of diagnosis) and age-matched control.
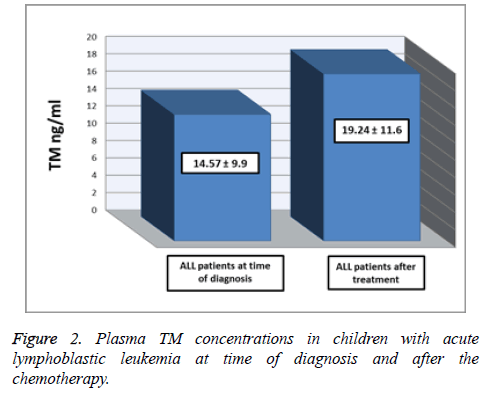
Table 2 and Figure 2 show the comparison between plasma TM concentrations in ALL patients at time of diagnosis and on day 35 of chemotherapy. The mean of plasma TM concentrations was significantly higher after chemotherapy (19.24 ± 11.6 ng/ml) than at time of diagnosis (14.57 ± 9.9 ng/ml), (p<0.05).
| Group | Mean ± SD | Number | Minimum | Maximum |
|---|---|---|---|---|
| ALL patients at time of diagnosis | 14.57 ± 9.9 ng/ml | 30 | 5.02 | 35.55 |
| ALL patients after the chemotherapy | 19.24 ± 11.6 ng/ml | 30 | 6.37 | 40.92 |
Table 2. Plasma TM concentrations between children with acute lymphoblastic leukemia at time of diagnosis and after the chemotherapy.
Discussion
It is well is known that TM is a sensitive indicator of vascular injury [12]. In this study plasma levels of sTM were determined as indexes of endothelial dysfunction in children with acute lymphoblastic leukemia.
Mean plasma TM levels done before chemotherapy were significantly higher in ALL patients than control. This is in agreement with Hatzipantelis et al. [12], Hagag et al. [13] and Hatzipantelis et al. [14], who found the significant increase of TM levels in the acute phase of the disease in children with ALL, and concluded that this confirms the serious dysfunction of the vascular endothelium, resulting from the disease itself.
Mean plasma TM levels were significantly higher in ALL patients after a chemotherapy compared to ALL patients at time of diagnosis. This is in agreement with Pascale Schneider et al. [15] who found the significant increase of TM levels during treatment of children with acute lymphoblastic leukemia. And Giordano et al. [16] who found that chemotherapy cause endothelial damage in children with ALL.
On the basis of our results, endothelial dysfunction in children with ALL at the time of diagnosis is likely caused by the disease itself whereas endothelial dysfunction during remission probably results from chemotherapy.
Conclusion
Plasma levels of sTM might represent a useful marker to evaluate the endothelial dysfunction that present during the acute phase of ALL. Longer term follow-up of the children of the present study and additional studies are required to confirm the potential prognostic value of these endothelial markers in children with ALL, and a better knowledge regarding the pathogenetic mechanisms behind the development of endothelial dysfunction and pharmacological strategies targeting endothelial dysfunction might be of great importance.
References
- Ribera JM, Oriol A. Acute lymphoblastic leukemia in adolescents and young adults. Hematol Oncol Clin North Am 2009; 23: 1033-1042.
- Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME. Long-term results of the childrens cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Childrens Oncology Group Report. Leukemia 2010; 24: 285-297.
- Li YH, Kuo CH, Shi GY, Wu HL. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci 2012; 19: 34.
- Ikezoe T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. Ikezoe J Intensive Care 2015; 3: 1.
- Martin F, Murphy R, Cummins P. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol 2013; 304: 1585-1597.
- Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol 2012; 34: 107-125.
- Adams TE, Huntington JA. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol 2006; 26: 1738-1745.
- Califano F, Giovanniello T, Pantone P. Clinical importance of thrombomodulin serum levels. Eur Rev Med Pharmacol Sci 2000; 4: 59-66.
- Lin FY, Tsai YT, Lee CY, Lin CY, Lin YW, Li CY, Shih CM, Huang CY, Chang NC, Tsai JC, Chen TL, Tsai CS. TNF-a-decreased thrombomodulin expression in monocytes is inhibited by propofol through regulation of tristetraprolin and human antigen R activities. Shock 2011; 36: 279-288.
- Hasing Chao T, Chuan Tsai W. Soluble thrombomodulin is a paracrine anti-apoptotic factor for vascular endothelial protection. Int J Cardiol 2014; 172: 340-349.
- Doroszko A, Jakubowski M, Niedzielska E. Elevated plasma ADMA contributes to development of endothelial dysfunction in children with acute lymphoblastic leukemia. Postepy Hig Med Dosw 2016; 70: 562-571.
- Hatzipantelis ES, Athanassiou MM, Gombakis N. Thrombomodulin and von Willebrand factor: relation to endothelial dysfunction and disease outcome in children with acute lymphoblastic leukemia. Acta Haematol 2011; 125: 130-135.
- Hagag A. Prognostic value of plasma levels of thrombomodulin and von Willebrand factor in Egyptian children with acute lymphoblastic leukemia. J Oncol Pharm Practice 2013; 1-6.
- Hatzipantelis ES, Athanasiou-Metaxa M, Tzimouli V. Dysfunction of the vascular endothelium in children with ALL. Arch Hellen Med 2008; 25: 771-780.
- Schneider P, Van Dreden P, Rousseau A. Increased levels of tissue factor activity and procoagulant phospholipids during treatment of children with acute lymphoblastic leukemia. Br J Hematol 2009; 148: 582-592.
- Giordano P, Molinari AC, Del Vecchio GC, Saracco P, Russo G, Altomare M. Prospective study of hemostatic alterations in children with acute lymphoblastic leukemia. Am J Hematol 2010; 85: 325-330.