ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Therapeutic effect of endostar combined with chemotherapy for colorectal cancer: An new evidence from meta-analysis
Hui Zhao1*, San Li Cai2 and Jing Feng Wang3
1Department of Thoracic Surgery-1, Jilin Cancer Hospital, 1018 Huguang Road, Changchun, Jilin Province, PR China
2Department of Thoracic Oncosurgery-3, Jilin Cancer Hospital, 1018 Huguang Road, Changchun, Jilin Province, PR China
3Center of Intervention, Jilin Cancer Hospital, 1018 Huguang Road, Changchun, Jilin Province, PR China
Accepted date: December 08, 2016
Objective: To compare the effect of Endostar in combination with chemotherapy for colorectal cancer with effect of chemotherapy alone.
Methodology: Take excluded criteria to select previous researches, evaluate the risk of bias and then propose the meta-analysis. The combined effect size is Odd Ratio (OR). If there is a negative result in the heterogeneity test, subjects can be combined by the fixed effects model, otherwise a sensitive test is proposed, negative sensitive researches cannot pass through into fixed effects model.
Results: Totally, there are 16 prospective cohort studies having a low risk of bias included in the metaanalysis. Compared with accepting chemotherapy alone, patients with Endostar therapy in combination with chemotherapy have significant improved outcomes, the probability of outcomes improvement increased 59 times(RR=1.59, 95% CI: 1.34,1.89). I2 value is 0%, indicating a low heterogeneity.
Conclusion: In conclusion, compared with chemotherapy alone, Endostar treatment of colorectal cancer in combination with chemotherapy has a better effect rather than chemotherapy alone based on RR +CR.
Keywords
Archenteric cancer, Drug, Evidence based review.
Introduction
Colorectal cancer is the most common cancer and is one of the most important causes of individual death around the world. The latest World Health Organization epidemiological data showed that in 2012, almost 8.2 million people worldwide died because of cancer, including 694,000 people died because of colorectal cancer. The detection rate was second high in both male and female patients [1]. In China, the colorectal cancer death ratio of male and female are 5.5% and 7.7%respectively among all cancer deaths [2]. Endostar (rh-endostatin, Shangdong Simcere-Medgenn Bio-Pharmaceutical Co., Ltd, China) is a recombinant of human endostatin. Plenty studies show that endostatin can be expressed through inducing endothelial cells apoptosis and inhibiting vascular endothelial growth factor (VEGF), and thus it plays an anti-angiogenesis role. VEGF is a pro-growth factor, with the promotion of the growth of blood vessels [3].
Endostar was used to aid the treatment of advanced non-small cell lung cancer at early stage. Short and long term observations showed it is an appropriate adjuvant therapy [4]. As one of anti-angiogenesis drugs, some small sample studies have reported the effect of endostatin in colorectal cancer. A systematic review contains 5 studies analyzed endostatin treatment of colorectal cancer effects. However, 80% of the similar meta - analysis was published before 2009 years, and the total sample of only 220 [5]. Thus, the initial studies did not provide sufficient evidence to prove the effect of Endostar in the treatment of colorectal cancer, including effectiveness for both rectum cancer and colon cancer treatment. Therefore, in order to assess the outcomes of Endostar treatment for colorectal cancer, and its safety issues, we conducted a comparative meta-analysis among clinical trials with the Endostar consolidated chemotherapy to make a comparison with these who take chemotherapy alone or placebo treatment to assess the impact of endostatin treatment.
Materials and Methods
Search strategy
We searched CNKI, WanFang, CQVIP, PubMed, The Cochrane library, Embase and Web of Science databases to retrieve clinical trials results. The searching key terms are: Endostar or recombinant human endostatin or YH-16and colon cancer or colon carcinoma or rectal carcinoma or rectal cancer or colorectal cancer or colorectal carcinoma. Publish date is restricted from July 2006 to August 2015.
Criteria for inclusion and exclusion
The target trials should be double-blinded, randomized, controlled trails. Sample participants of these trails are proposed to be patients of colorectal cancer, including rectum cancer and colorectal cancer. These metastatic cancer, whose primary lesions were colorectal cancer and remains their primary lesions are also involved. Content of experimental evaluation is the comparison of Endostar or Endostar combined with chemotherapy and chemotherapy alone or placebo treatment. Outcomes of treatment are categorized as complete remission (CR), stable disease (SD), partial release (PR), disease development (PD), relative rate (RR: CR+PR) and clinical benefit rate (CBR: CR+PR+SD). The final document inclusion and exclusion is determined by two experts, which are oncology clinical experts and epidemiologists respectively. When they hold different opinions, the third person, who is the author, could ultimately determine inclusion or exclusion of the results.
Data extraction and assessment of risk of bias
From clinical researches, we extracted data including gender, age, colorectal cancer types, interventions and treatment outcome. Then we did risk assessment for all researches according to the evaluation of risk of bias tool provided by Cochran Collaboration Web. Each risk assessment is based on six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting data with other topics. Each entry for every domain has three judgments result: yes, representing low risk; "unclear" representing not know or unclear risk of bias; no, representing a high risk. Results of the assessment would be expressed by the risk of bias bar chart, drawn by Revman software.
Heterogeneity test and combined effect size
We judge whether there is heterogeneity between studies according to the L'Abbe Figure and I2 values. In L'Abbe figure, if each point represents the research is too far away from the OR straight, it suggests that the heterogeneity exists. I2 value ≤ 25%, it suggests that there is a low heterogeneity between studies, I2 value ≤ 50% and ≥ 25% suggests a moderate heterogeneity between studies, I2 value>50% suggests a high heterogeneity. Based on L'Abbe and I2 value, if there is heterogeneity between studies then we take meta regression analysis to identify sources of heterogeneity, and redefine the research literature, once meta regression analysis cannot identify the source of the heterogeneity, we choose random effects model to combine effect size. If there is no heterogeneity, then we combine effect size through the fixed effects model. Relative Risk (RR) and 95% confidence intervals are used to assess the degree of Endostar or its combination with chemotherapy drugs effect in the treatment of colorectal cancer. Statistics related to the P values is estimated by bidirectional estimation, and 0.05 is the statistical cutoffs. The data was analyzed by the software of stata.
Results
Search results
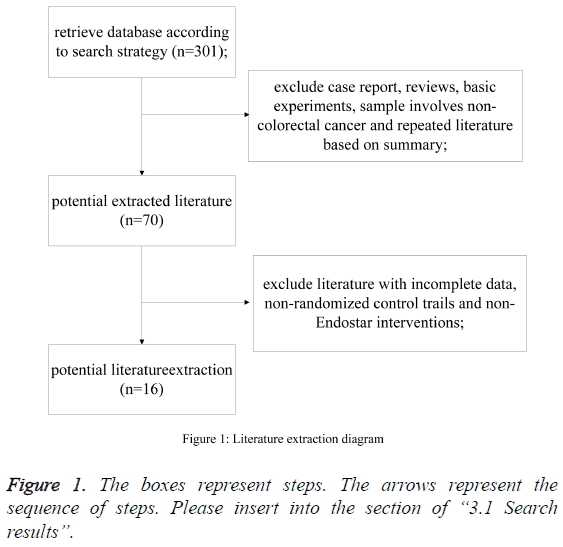
According to the search strategy, inclusion and exclusion criteria, 16 randomized controlled trials were eligible for our analysis [6-21]. According to the search strategy, 301 studies were selected. By reading a summary, we excluded reports, reviews, and basic research papers. After screening, 16 studies were left, through a summary screening, to form systematic review (Figure 1).
Characteristics of the participants
From 16 clinical trials, we got 723 research participants’ research data, including gender, age, colorectal cancer types, interventions and treatment outcome and so on. Among them, the chemotherapy regimen of XELOX, FOLFOX4, FOLFOX are 4, 4, and 2 respectively. Participants aged from 18 to 80 years old. The major metastasis is liver metastases. Baseline characteristics of participants are described in Table 1.
| ID | Study | Year | Chemotherapy | Endostar Therapy1 | Therapy | Follow(m)2 | Sex M/F | Age | Metastasis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Guozhong Li | 2009 | XELOX | -,1~14d,7d,repeat cycle | ≥ 1 cycle | - | 35~75 | Liver, lung | |
| 2 | Hong-Xia Xu | 2011 | FOLFOX4 | 15mg/d,1~14d,-, repeat cycle | ≥ 2 cycle | - | |||
| 3 | Shimin Ping | 2013 | Irinotecan/Oxaliplatin | 15mg/d,1~14d,7d,repeat cycle | ≥ 2 cycle | 6~24m | 1.765277778 | 18~75 | Liver |
| 4 | Dong Yan | 2011 | XELOX | 15mg/d,1~14d,7d,rpeat cycle | ≥ 2 cycle | >1m | 1.807638889 | 65~79 | Liver, lung, bone |
| 5 | Li Ding | 2009 | FOLFOX4 | -,1~14d,-,rpeat cycle | ≥ 2 cycle | >1m | 1.218055556 | 39~74 | Liver |
| 6 | Rongrong Zhang | 2009 | FOLFOX4 | -,1~14d,-,rpeat cycle | 4~8cycle | >1m | - | 38~70 | - |
| 7 | Lin Lai | 2014 | New radiotherapy | -,1~14d,7d,rpeat cycle | 2 cycle | - | 0.756944444 | 31~69 | None |
| 8 | Haipeng Wang | 2012 | FOLFOX | 15mg/d,1~14d,-,repeat cycle | 4 cycle | - | 1.430555556 | 62~75 | - |
| 9 | Zhuo Liu | 2014 | FOLFIRI | -,1~14d,-,repeat cycle | 4 cycle | - | 1.515277778 | 43~73 | Liver |
| 10 | ZhongzhiJia | 2012 | - | 10~20mg/d,1~14d,7d,repeat cycle | - | 5~12m | 1.135416667 | 37~72 | Liver |
| 11 | BiaosenLuo | 2012 | FOLFOX | 15mg/d,1~14d,-,rpeat cycle | ≥ 2 cycle | - | - | ≥ 18 | - |
| 12 | Lizhen Zhang | 2013 | Irinotecan/Oxaliplatin | -,1~14d,7d,repeat cycle | ≥ 2 cycle | 6~24m | 1.765277778 | 18~75 | - |
| 13 | Chuanqi He | 2009 | FOLFOX4 | -,1~14d,7d,repeat cycle | 3~6 cycle | >1m | 1.345833333 | 18~70 | - |
| 14 | Jing Liu | 2009 | IFL | 15mg/d,1~14d,-,repeat cycle | 2~4 cycle | >1m | 2.008333333 | 36~62 | Liver, lymph |
| 15 | Yan Lin | 2012 | XELOX | -,1~14d,-,repeat cycle | 2~8 cycle | - | 1.684027778 | 43~80 | Liver, lung |
| 16 | YitaoJia | 2011 | XELOX | 15mg/d,1~14d,7d,rpeat cycle | ≥ 2 cycle | - | 1.1 | 39~65 | - |
2. Follow-up time. “m” means month.
Table 1: 16 clinic trails’ participants baseline characteristics.
Bias risk assessment
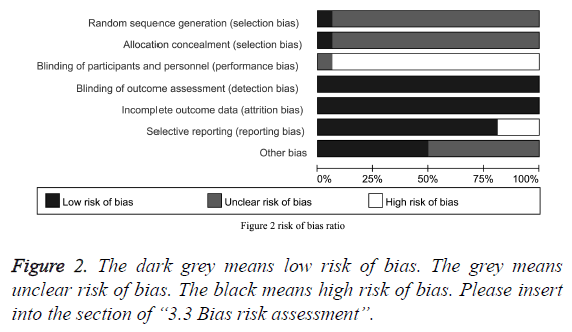
Risk of bias assessment shows that, in terms of random sequence generation and allocation concealment, more than 90% of the study is not clear their risk of bias. In terms of binding of participants and personnel, the existence of high risk of bias appears in more than 90% of studies. Lower risk of bias appears in terms of income outcome data and blinding of outcome assessment. In terms of selective reporting bias, less than 25% of the study has a low risk. In addition, we believe that there is potential interest relationship between researchers and drug manufacturers, and hence whether manufactures were informed consent for other similar researches is another important source of bias. The evaluation results indicated that 50% of the studies fail to insure the risk of bias. However, because classification of the therapeutic effect is to be determined based on the results of imaging studies, subjects and investigators were blinded during trails or not has minimal impact on determining the results. Therefore, we believed the involved 16 studies generally are not at a high risk of bias (Figure 2).
Endostar for adjuvant therapy of colorectal cancer
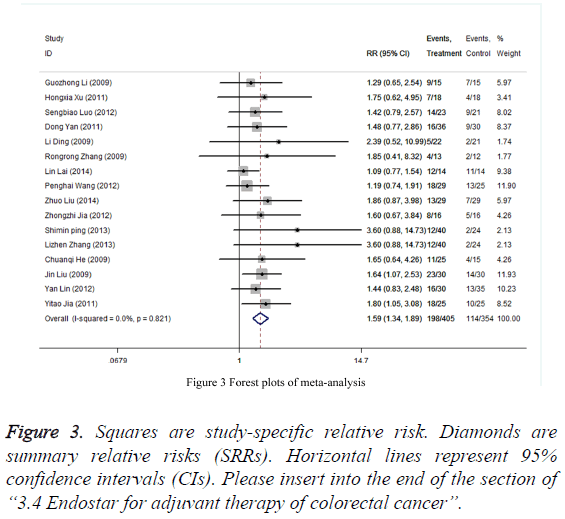
Forest plots of the fixed effects model shows that, compared with patients receiving chemotherapy alone, patients with Endostar therapy in combination with chemotherapy in patients have a significant improved outcomes (CR + PR), with 59% increased of improvement probability (RR=1.59, 95% CI: 1.34,1.89). I2 is 0%, suggesting the presence of a low heterogeneity in the studies extraction in the meta-analysis. It can be initially estimated that all participants come from the same population (Figure 3). Therefore, the fixed effects model effect size used above is appropriate.
Publication bias test
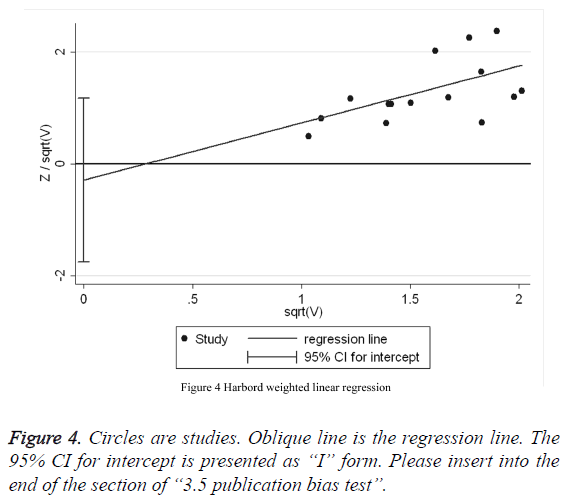
Harbord weighted linear regression (Table 2) reveals that there is a absence of publication bias among the 16 studies (t=1.20, p=0.25, 95% CI: -0.07, 0.24). Meanwhile, Harbord weighted linear regression (Figure 4) shows that almost regression line go through the 0 pot, suggesting that the meta-analysis of the literature in this analysis has a pretty low publication bias.
| Coef. | Std. Err. | t | P value | 95% CI | ||
|---|---|---|---|---|---|---|
| Slope | 2.77 | 0.03 | 80.64 | 0 | 2.69 | 2.84 |
| Bias | 0.09 | 0.07 | 1.2 | 0.25 | -0.07 | 0.24 |
Table 2: Harbord weighted regression.
Discussion
Our research found, compared with chemotherapy alone in the treatment of colorectal cancer, endostar combined with chemotherapy has a significant improved effect (based on CR +RR). Moreover, there is no significant heterogeneity as well as publication bias among those 16 propective cohort studies included in this research. The metastasis and growth of solid tumor depend on its induced ability of new blood vessels in the tumor. Endostar, as a recombinant human endostatin, and also due to that endostatin presents targeting effect for vascular endothelial cells and vascular endothelial growth, is now known as one of the most effective anti-angiogenic agents. Findings from Phase III clinical reports indicates that after at least six weeks of Endostar combined with vinorelbine, cisplatin treatment, the efficient treatment of advanced nonsmall cell lung cancer have significantly improved, while within 6 months, the median tumor progression time is significantly suspended [22]. A three-year fellow-up visit of these patients reveals that the average median survival time of combined treatment is 6.3 months while control group received chemotherapy alone is only 3.6 months [4].
Endostar not only could control advanced non-small cell lung cancer, animal experiments and clinical trials show that it also works for colorectal cancer. Zhang [23] reported the Endostar by loaded ultrasonic micro-bubble could effectively reduce the expression of vascular endothelial growth factor of colorectal cancer in mouse experiments, meanwhile, it can also increase tumor cells’ apoptosis and tumor ultra structure’s degradation. Jia found that in a in a mouse xenograft model, Endostar can inhabit tumor growth and the rate of lymph node metastasis by suppressing angiogenesis and lymph angiogenesis. The expression of VEGF-A, -C and -D in cells of Endostar treatment was substantially lower than that of controls [24]. Amounts of studies have reported the same results, Endostar loaded nanoparticle carriers or anti-B powder jointed with Endostar also showed the inhibitory effect on the growth of colorectal cancer cells [25].
However, there are still some limitations in this research. First, in term of the sample source, the participants were Asian populations, the difference of Endostar and chemotherapy joint treatment and chemotherapy treatment alone for colorectal cancer is not clear if it applies to Europe, America, Africa, Australia and other people. Senond, in terms of confounding factors, the study collected 16 studies which do not contain accurate information about the age and gender mostly, we are unable to estimate the difference between chemotherapy combined Endostar treatment and chemotherapy treatment among colorectal cancer patients with different demographic characteristics. Third, when talking about the treatment effect, the outcome is based on CR+RR, we did not collect enough other data such as survival time, symptoms, imaging results and the variety of the laboratory indexing. To an extent, whether CR+RR is enough for judging the outcome is not clear. Therefore, when we draw the conclusion that endostar combined with chemotherapy has a significant improved effect on colorectal cancer, we should be more careful. In order to improve the study, comprehensive, quality systematic reviews and meta-analysis are needed worldwide with multi-centers cooperation to study multi-human races.
References
- Li X. Tes, a potential Mena-related cancer therapy target. Drug DiscovTher 2008; 2: 1.
- http://www.who.int/cancer/country-profiles/chn_en.pdf?ua=1
- Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J ClinOncol 2001; 19: 1207-1225.
- Sun Y, Wang JW, Liu YY, Yu QT, Zhang YP, Li K. Long-term results of a randomized, double-blind, and placebo-controlled phase III trial: Endostar (rh-endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non-small cell lung cancer. Thoracic Cancer 2013;4: 440-448.
- Pan Y, Jiao G. Short-term therapeutic effect of Endostar combined with chemotherapy for advanced colorectal cancer: a meta-analysis. Nan Fang Yi Ke Da XueXueBao 2014; 34: 270-274.
- Xu HX, Huang XE, Qian ZY, Xu X, Li Y. Clinical observation of Endostar® combined with chemotherapy in advanced colorectal cancer patients. Asian Pac J Cancer Prev 2011; 12: 3087-3090.
- Chuanqi H, Zheng W, Jinxian C. Controlled clinical studies of recombinant human endostatin combined with FOLFOX4 treatment of advanced colorectal cancer. Gastroenteropathy 2009;08: 453-457.
- Tao JY, Yin RC, Lu YX, Fang LC, HuizX, Xin LZ. Clinical observation of treatment of advanced colorectal cancer by recombinant human endostatin combined with chemotherapeutics. Clinical Meta Anal 2011;4: 332-334.
- Zhi JZ, Qin LS, Feng T, Wei ZJ, Kai W, Yun W. Recombinant human endostain inhibition in combination with hepatic artery embolism chemotherapy treatment of clinical application of postoperative colorectal cancer liver metastases. J Practical Oncol 2012;2: 148-151.
- Lin L, Quan CJ, Dian C. Endostar combined with radiotherapy in new low ?-? stage colorectal cancer. Modern Oncol 2014;1: 115-117.
- Zhong LG, Qun LH, Qi ZL, Yin X, Bo WH, Guang SW. Clinical investigation of recombinant human endostatin combined with XELOX treatment of advanced colorectal cancer. Chinese J ClinOncol 2009; 12: 688-689.
- Yan L, Hua YS, Min L. Clinical observation of recombinant human endostatin combined with chemotherapy in treatment of advanced colorectal. Practical Clin Med 2012;11: 114-116.
- Jin L. Clinical analysis of 30 cases with advanced colon cancer treated by IFL combined with recombinant human endostatin.Chinese J Coal Industry Med 2009;12: 8.
- Zhuo L, Shan Z, Pin LY, Jia AX, Pin X, Gang WH. Clinical observation of patients with colon cancer treated by FOLFIRI combined with recombinant human endostatin. Progress Modern Biomedi 2014;22: 4265-4268.
- Biao LS. Clinical observation of 25 patients with colon cancer treated by Endostar. China Pharmaceuticals 2012;23: 71-73.
- Min PS, Xia GL, Li W. The efficacy of recombinant human endostatin combined with chemotherapy in the treatment of colorectal cancer liver metastases. Basic Med Forum 2013;1: 21-22.
- Pen WH, Jia ZX, Lin TX. Capecitabine, oxaliplatin combined with recombinant human endostatin therapy for elderly patients with advanced colorectal cancer. Chinese J Gerontol 2012;16: 3542-3543.
- Dong Y, Hong D. Clinical observation of endostar joint first-line XELOX treatment of advanced colorectal cancer. Chinese J Cancer Prevention Treatment 2011;12: 961-964.
- Zhen ZL, Hong L. Clinical observation of treatment of patients with advanced colorectal cancer by recombinant human endostatin combined with chemotherapy. Strait Pharmaceutical J 2013;1: 222-223.
- Rong ZR, Shu LM, Yu TS, Li Z, Yi K, Kai Z. Study of treatment of endostatin combined with chemotherapy for advanced colorectal cancer clinical Shenzhen. J Integrated Traditional Chinese Western Med 2009;6: 339-341.
- Li D. clinical studies of treatment of endostar combined with chemotherapy for advanced colorectal cancer. Sino-foreign health abstract 2009;20: 6-7.
- Wang J, Sun Y, Liu Y, Yu Q, Zhang Y. [Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients]. Zhongguo Fei Ai ZaZhi 2005; 8: 283-290.
- Zhang C, Huang P, Zhang Y, Chen J, Shentu W. Anti-tumor efficacy of ultrasonic cavitation is potentiated by concurrent delivery of anti-angiogenic drug in colon cancer. Cancer Lett 2014; 347: 105-113.
- Jia Y, Liu M, Huang W, Wang Z, He Y, Wu J. Recombinant human endostatinendostar inhibits tumor growth and metastasis in a mouse xenograft model of colon cancer. PatholOncol Res 2012;18: 315-323.
- Qian X, Yan B, Zhou X, Xie L, Wei J, Li R. Synergistic antiangiogenic activity of tetrandrine combined with Endostar on the human umbilical vein endothelial cell model. Cancer BiotherRadiopharm 2013;28:385-390.