ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Review Article - Biomedical Research (2017) Volume 28, Issue 3
Treated dentin matrix combined with dental follicle cells as a promising approach for tissue engineered tooth root regeneration
Department of Stomatology, the Affiliated Hospital of Hainan Medical University, No. 31, Longhua Road, Haikou 570102, Hainan Province, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Genjian Zheng
Department of Stomatology the Affiliated Hospital of Hainan Medical University, PR China
Accepted on August 05, 2016
Tissue-engineered tooth regeneration is an effective treatment of tooth loss. Tissue engineering strategies to construct a tooth employ interactions between seeding cells, scaffolds, and inductive microenvironments. However, there are still no suitable scaffolds to restore the tooth and the surrounding normal tissue structure. Treated Dentin Matrix (TDM) is a calcified tissue which makes up most of the tooth and becomes a key component for tissue engineering of tooth structure. TDM is available in abundance, can easily be trimmed to a variety of shapes, and has no obvious immune rejection. TDM scaffold combined with dental follicle cells (DFCs) could be implanted into an inductive alveolar fossa microenvironment to regenerate the tooth root. We propose that TDM scaffold combined with DFCs provides a new approach for the treatment of tooth loss.
Keywords
Treated dentin matrix, Dental follicle cells, Tissue-engineering, Tooth root, Regeneration.
Introduction
Loss of permanent dentition is a common condition that has a negative effect on a patient’s overall quality of life. The common causes for tooth loss include caries, periodontal disease and accidents. Conventionally, lost teeth are replaced with dentures or synthetic dental implants. However, none of those options can restore a normal tooth structure and completely repair masticatory function.
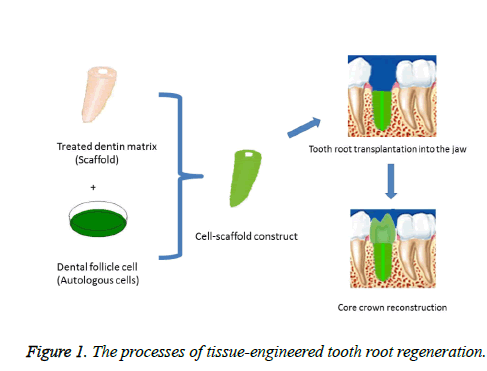
A tissue-engineered tooth is made possible by harvesting seeding stem cells from the patient, obtaining a suitable scaffolding material for cell growth and inducing the stem cells toward a relevant lineage, and then reintroducing cell-scaffold construct to the patient's inductive alveolar fossa microenvironment to reconstruct the tooth and the surrounding normal tissue structures (Figure 1) [1-4]. During tissueengineered tooth regeneration, the tooth root is primarily regenerated while the crown is restored with traditional prosthodontic treatment. This strategy not only restores a patient's appearance and masticatory function, but also is effective to treat tooth loss. Commonly used cell scaffolding materials include Ceramic Bovine Bone (CBB), nanostructured materials, the copolymer of oly-DL-lactide and glycolide (PLG), polyglycolic acid (PLGA), polysaccharide and hydroxyapatite/tricalcium phosphate (HA/TCP) [3,5-9]. These materials typically provide only structural support for cells but could not provide an environment conducive to cell differentiation relevant to tooth regeneration. There is an abundance of bioactive molecules, factors, and proteins related to pulp and dentin tissue formation in dentin matrix, such as dentin sialoprotein (DSP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), type I collagen (COLI), alkaline phosphatase (ALP) and so on [10]. Treated dentin matrix (TDM) scaffold with inductive properties may offer specific benefits in achieving simultaneous regeneration of periodontium/pulp-dentin complex and constraining the final shape of tissue engineered tooth [11-13].
Treated Dentin Matrix Scaffold and Tooth Root Regeneration
TDM is a calcified tissue which makes up most of the tooth and becomes a key component for tissue engineering of tooth structure. TDM is able to induce the differentiation of dental follicle cells (DFCs) into odontoblast lineage due to the signaling molecules such as bone morphogenetic protein 2 and transforming growth factor beta (TGF-β) inside TDM. TDM is non-cytotoxic, bioactive, and capable of providing a threedimensional microenvironment supporting cell growth, odontogenic differentiation, and cellular organization into the structure of the tissue. TDM scaffold is available in abundance from human premolars and can be trimmed easily to a variety of shapes according to the patient’s jaw and occlusion. Due to decellularization, there is no obvious immune rejection after TDM implantation in the host [13].
Natural tooth root structures include dental pulp (DP), odontoblasts layer, dentin (DE), cementum (CE), periodontal ligament (PDL) inserting into CE and alveolar bone (AB). TDM from human or rat has recently been shown to be a suitable scaffold for dentin regeneration [11-14]. Implantation of human TDM combined with DFCs in vivo for 8 weeks in a nude mouse model led to complete regeneration of dentin tissues, which expressed the dentin markers DSP and DMP-1 (regarded as the markers of odontoblasts) [1]. This study showed that TDM is an ideal biomaterial for human dentin regeneration. In another study murine DFCs were seeded onto immunocompetent murine TDM and further incubated for 1-2 weeks in vitro and for 2-4 weeks in vivo. In vitro, in addition to DSP and DMP1 , DFCs induced by TDM expressed osteocalcin (OCN), DSP, COLI, osteopontin (OPN), osteonectin (ON) and ALP, all of which are expressed by odontoblasts. In vivo, TDM induced the regeneration of dentin, which expresses DSP and DMP-1.
These results showed the successful regeneration of complete and prefabricated-shaped dentin [13]. Upon transplantation of allogenic rat TDM combined with DFCs into immunocompetent rats for four weeks, tooth root-like tissues stained positive for markers of dental pulp and periodontal. These tissues were regenerated in the alveolar fossa, but not in the skull and omental pockets. These results confirm that the combination of TDM with DFCs in the alveolar fossa is a feasible strategy for tooth root regeneration [14]. During implantation, the alveolar bone should be prepared appropriately to fix TDM scaffold. Fresh alveolar fossa is adopted as an inductive microenvironment for the regeneration of PDL/CE complex [15]. TDM shows excellent biocompatibility and bioactivity due to the decellularization and reserve of an abundance of bioactive factors. Furthermore, the success of cryopreservation of TDM provides a tissue engineering scaffold that is readily available for patient treatments [16]. Thus TDM is an ideal biomaterial for human tooth root regeneration.
Dental Follicle Cells and Tooth Root Regeneration
Non-dental stem cells including bone marrow mesenchymal stem cells [17], adipose-derived stem cells [18], embryonic stem cells [19], and induced pluripotent stem cells (iPS) [20] have been shown to induce periodontal regeneration. These cells have the potential of differentiating into osteoblasts, cementoblasts and fibroblasts, and can form cementum/PDLlike structures under appropriate conditions. The dental follicle (DF) is a loose connective tissue surrounding the unerupted tooth and plays crucial role in tooth eruption by anchoring the tooth in its socket to the surrounding alveolar bone. DF is present in human impacted teeth, which is commonly extracted and disposed as medical waste in dental practices. DF is also easily obtained from clinically discarded third molar extractions. DFCs isolated from DF possess mesenchymal stem cell (MSC)-like qualities, including the capacity for selfrenewal and multi-lineage differentiation including adipogenesis, osteogenesis, neurogenesis, chondrogenesis, and thus form pulp-dentin complex and PDL/CE-like tissue [14,21-24].
DFCs are the progenitors of osteoblasts and periodontal ligament cells (PDLCs) within dental follicle. Under certain conditions, DFCs can be induced to differentiate into a variety of cells such as chondrogenic, osteogenic, adipogenic and neuronal cells. In particular, DFCs have significant advantages for periodontal tissue regeneration over PDLCs or other dentalderived cells. Furthermore, human DFCs can be easily isolated from discarded wisdom tooth, and thus are the ideal cell source for tooth regeneration [14,21]. DFCs isolated from human impacted molars were combined with ceramic bovine bone and implanted into subcutaneous pockets of nude mice. The formation of a PDL/CE complex was observed six weeks after implantation, indicating that heterogeneous DFCs contribute to the formation of PDL/CE complex [21,25].
Dental follicle cell sheets (DFSCs) were harvested from the third molars of six-month-old pigs and expanded in vitro. Primary dental pulp cells, primary enamel organ epithelial cells and subcultured DFSCs were then successively added to mimic the tooth primordia. The combination was inserted into a cavity inside the shaft of the bone and later transplanted into the omentum of six-week-old immunocompromised rats. After 24 weeks, large amount of regenerated dentin was observed and odontoblasts were present along the formed dentin. A thick layer of cementum-like tissue covered the newly formed dentin and the space between the regenerated dentin and bone was filled with connective tissues. Collagen fiber-like tissues resembling PDL were perpendicularly attached to the cementum-like layer. However, collagen fiber-like tissues were not observed on the surface of the cementum with any bone anchorage [26]. DFCSs also improved the survival rate of implanted cells for dentinogenesis and tooth root construction. In vitro, after TDM treatment DFCSs highly expressed DMP-1 and BSP, indicating the potential for odontogenesis and osteogenesis. In vivo, TDM could induce and support DFCs to develop a new pulp-dentin complex and PDL/CE-like tissues that were positive for markers such as DSP, nestin, VIII factors, COLI and cementum attachment protein (CAP), indicating successful tooth root regeneration [27]. Taken together, these results suggest that DFCs are suitable seeding cell for pulp-dentin complex and PDL/CE-like tissue regeneration which are essential components of tooth root.
In conclusion, the combination of TDM with DFCs in the induced alveolar fossa may be a promising approach for tissueengineered tooth root regeneration, which provides an effective treatment for tooth loss.
References
- Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010; 31: 4715-4724.
- Angelova-Volponi A, Kawasaki M, Sharpe PT. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J Dent Res 2013; 92: 329-334.
- Mitsiadis TA, Woloszyk A, Jiménez-Rojo L. Nanodentistry: combining nanostructured materials and stem cells for dental tissue regeneration, Nanomedicine (Lond) 2012; 7: 1743-1753.
- Horst OV, Chavez MG, Jheon AH, Desai T, Klein OD. Stem cell and biomaterials research in dental tissue engineering and regeneration. Dent Clin North Am 2012; 56: 495-520.
- Holtgrave EA, Donath K. Response of odontoblast-like cells to hydroxyapatite ceramic granules. Biomaterials 1995; 16: 155-159.
- Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials 2007; 28: 680-689.
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 2003; 82: 976-981.
- Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. Bioengineered dental tissues grown in the rat jaw. J Dent Res 2008; 87: 745-750.
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81: 531-535.
- Park ES, Cho HS, Kwon TG, Jang SN, Lee SH, An CH. Proteomics analysis of human dentin reveals distinct protein expression profiles. J Proteome Res 2009; 8: 1338-1346.
- Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cell in the dental follicle. J Dent Res 2008; 87: 767-771.
- Li R, Guo W, Yang B, Guo L, Sheng L, Chen G. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011; 32: 4525-4538.
- Guo W, He Y, Zhang X, Lu W, Wang C, Yu H. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009; 30: 6708-6723.
- Guo W, Gong K, Shi H, Zhu G, He Y, Ding B. Dental Follicle Cells and Treated Dentin Matrix Scaffold for Tissue Engineering the Tooth Root. Biomaterials 2012; 33: 1291-1302.
- Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T. Fully functional bioengineered tooth replacement as an organ replacement therapy. ProcNatlAcadSci USA 2009; 106: 13475-13480.
- Jiao L, Xie L, Yang B, Yu M, Jiang Z, Feng L.Cryopreserved dentin matrix as a scaffold material for dentin-pulp tissue regeneration. Biomaterials 2014; 35: 4929-4939.
- Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. JPeriodonto 2004; l75: 1281-1287.
- Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A 2008; 14: 945-953.
- Inanç B, Elçin AE, Unsal E, Balos K, Parlar A, Elçin YM. Differentiation of human embryonic stem cells on periodontal ligament fibroblasts in vitro. Artif Organs 2008; 32: 100-109.
- Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol 2011; 226: 150-157.
- Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Eng Part A 2012; 18: 459-470.
- Sujesh M, Rangarajan V, Ravi Kumar C, Sunil Kumar G. Stem cell mediated tooth regeneration: new vistas in dentistry. J Indian ProsthodontSoc 2012; 12: 1-7.
- Zivkovic P, Petrovic V, Najman S, Stefanovic V. Stem cell-based dental tissue engineering. ciWorldJ 2010; 10: 901-916.
- Rodríguez-Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramírez MD, Meseguer L. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal2012; 17: e1062-e1067.
- Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev 2010; 19: 1405-1415.
- Honda MJ, Tsuchiya S, Shinohara Y, Shinmura Y, Sumita Y. Recent advance in engineering of tooth and tooth structure using postnatal dental cells. Jpn Dent Sci Rev 2010; 46: 54-66.
- Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012; 33: 2449-2461.