ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Short Communication - Biomedical Research (2017) Volume 28, Issue 7
UHPLC/MS detection of yohimbine in food supplements
1Faculty of Pharmacy, Department Pharmacognosy and Pharmaceutical Chemistry, Medical University, Plovdiv, Vasil Aprilov Blvd, 15-A, Bulgaria
2Faculty of Pharmacy, Department of Pharmaceutical Sciences, Medical University, Plovdiv, Vasil Aprilov Blvd, 15-A, Bulgaria
3Faculty of Pharmacy, Department of Social pharmacy and Pharmacoeconomics, Medical University, Sofia, Dunav 2nd Street, Bulgaria
- *Corresponding Author:
- Stanislava Ivanova
Faculty of Pharmacy
Department Pharmacognosy and Pharmaceutical Chemistry
Medical University, Plovdiv, Bulgaria
Accepted on January 4, 2017
UHPLC in combination with electrospray ionization mass spectrometric detection in the positive ion mode has been used successfully to screen the yohimbine content in food supplements. The chromatographic behaviour of yohimbine is examined using Accela UHPLC with mass spectral detection HRMS “Q-Exative” with H-ESI-interface (“Thermo Fisher Scientific”, Waltham, MA, USA) and columns “Kynetex” RP C8 50 × 3 mm × 2.6 μm type “core-shell” and “Poroshell” RP CE18 150 × 3 mm × 2.7 mm. We have used mobile phase containing: acetonitrile, methanol water, ammonium formate. We have made series of chromatograms in positive mode of ionization and in negative mode of ionization. We have found that the ionization in positive mode offers a significantly higher sensitivity and also higher selectivity than the negative ion mode. This analytical method could be successfully applied for analytical control of food supplements containing yohimbine.
Keywords
Yohimbe, Yohimbine, Pausinystalia yohimbe, Food supplement, HPLC.
Introduction
Yohimbine is an alkaloid derived from the African plant Pausinystalia yohimbe (Rubiaceae). Pausinystalia johimbe is a tree native to the coastal forests of Central Africa and is distributed from South East Nigeria to the Congolese Mayombe [1]. Its bark contains up to 6% of a mixture of alkaloids. The bark of the plant has been traditionally used in folk medicine as general tonic, performance enhancer and as an aphrodisiac [2-6]. The pharmacological researches on yohimbine have been started since 1949 [7,8]. It has been found that Yohimbine antagonizes a-2 adrenergic receptors. Nowadays yohimbine is used as an active ingredient in many food supplements. These products are promoted to improve sexual and athletic performance and also for weight loss. The daily intake of yohimbine can cause many adverse effects like headache, nausea, increased urinary urge; insomnia, anxiety, restlessness, irritability, increase of blood pressure and pulse rate, palpitation, dizziness, vomiting, anorexia, gastric complaints, flush, sweating, shivering, allergic reactions, nervousness, hypotension, tremor, bronchospasm, dysuria, decreased urge, genital pains, exanthema. For this reason in several European countries (e.g. United Kingdom, Ireland, Netherlands, Belgium, Denmark, Czech Republic) as well as in Canada, Australia and New Zealand the inclusion of yohimbine in food supplements or foods is prohibited [9]. In the most part of World yohimbine products are distributed as food supplements.
For manufacturing process yohimbine can either be obtained from the bark or chemically synthesised. Dried bark from the twigs and trunk of the Pausinystalia yohimbe is used in its entirety, cut up or ground into powder to be used as pharmaceutical products. A real problem for the consumers’s safety is that there is no information on standardisation of the extracts for use in food supplements with regard to the ratio of extracted material to starting material, extraction solvent, and content of biologically active ingredients [9]. In many food supplements the qualitative and quantitative composition of their components is not declared. According European Pharmacopoeia yohimbine hydrochloride should be kept in air tight containers, protected from light. In fact no data are available on the stability of Yohimbe bark or preparations such as extracts thereof in food supplements. The strong analytical control of these products is essential for customer safety. Unfortunately the analytical control is not obligatory for the food supplements. A variety of techniques have been used successfully to measure yohimbine including: GC-MS, HPLC with UV, fluorescence or amperometric detection [10-14] and also TLC determination but there are no official inter- laboratory validated methods for determining the levels of yohimbine and accompanying alkaloids in food supplements.
Materials and Methods
Materials
І) Reference standard: Yohimbine with purity>98% (Sigma Aldrich).
II) Reagents: Acetonitrile for HPLC ≥ 99.93% (Sigma Aldrich); Methanol for HPLC ≥ 99.9% (Sigma Aldrich); Ammonium formate for HPLC ≥ 99.0% (Sigma Aldrich), distilled water.
III) Samples: Samples were purchased from dietary supplement stores and pharmacies.
Sample 1-capsules 500 mg. Content: yohimbine 500 mg.
Sample 2-capsules 800 mg. Content: L-arginine 250 mg, Yohimbe extract 50 mg (10% extract=5 mg yohimbine), ginseng 150 mg, Tribulus terrestris 150 mg.
Sample 3-capsules 650 mg. Content: Yohimbe 50 mg (10% extract=5 mg yohimbine); Ginko biloba extract-40 mg; Vitamin E-40 mg; Vitamin B6-10 mg.
Methods
UHPLC/MS method: I) Instrumentation: “Accela UHPLC” with mass spectral detection HRMS “Q-Exative” with H-ESI-interface (“Thermo Fisher Scientific”, Waltham, MA, USA) and columns “Kynetex” RP C8 50 × 3 mm × 2.6 μm type “core-shell” and “Poroshell” RP CE18 150 × 3 mm × 2.7 μm type “core-shell”; ultrasonic bath (Branson Wilmington, NC, USA); apparatus for ultra-pure water: “Milli-Q”, “Milipore” (Bed-ford, MA, USA) and “Elga” (VWR International, Randor, PA, USA).
II) Chromatographic conditions: Mobile phase A: 10% acetonitrile/5% methanol/ 75% water
Mobile phase B: 30% acetonitrile/20% methanol/50% water
Mobile phase C: 0.2 M ammonium formate, pH=6.4
Gradient: from 95% A/5% B to 95% C/5% B over 10 min linear gradient;
15 min 95% B/5% C, isocratic;
5 min 95% A/5% C, isocratic.
III) Sample preparation: Standard solution of yohimbine: 5.0 mg of referent substance yohimbine with purity>98% (Sigma Aldrich) was weighed in a volumetric flask (10 ml). The substance was dissolved in 5 ml of the component “B” of the mobile phase and sonificated in an ultrasonic bath for 5 min. We have added to the solution 5 ml of component “A” of the mobile phase. The obtained standard solution has a concentration of 500 μg/ml and is stable upon storage in a refrigerator (4 ± 8°C) for more than 10 days. From the basic standard solution by dilution with component “A” of the mobile phase we have obtained working standard solutions with a concentration of 200 μg/ml, 100 μg/ml, 20 μg/ml, 10 μg/ml, 2 μg/ml, 1 μg/ml, 0.2 μg/ml and 0.02 μg/ml.
Sample 1 solution: 20 mg of the contents of the capsule are dissolved in 250 ml acetonitrile/water (the obtained solution has a concentration of 80 μg/ml);
Sample 2 solution: 37 mg of the contents of the capsule are dissolved in 250 ml acetonitrile/water (the solution has a concentration of 148 μg/ml.
Sample 3 solution: 82 mg of the content of the tablet is dissolved in 250 ml acetonitrile/water (the solution has a concentration of 328 μg/ml).
Results and Discussion
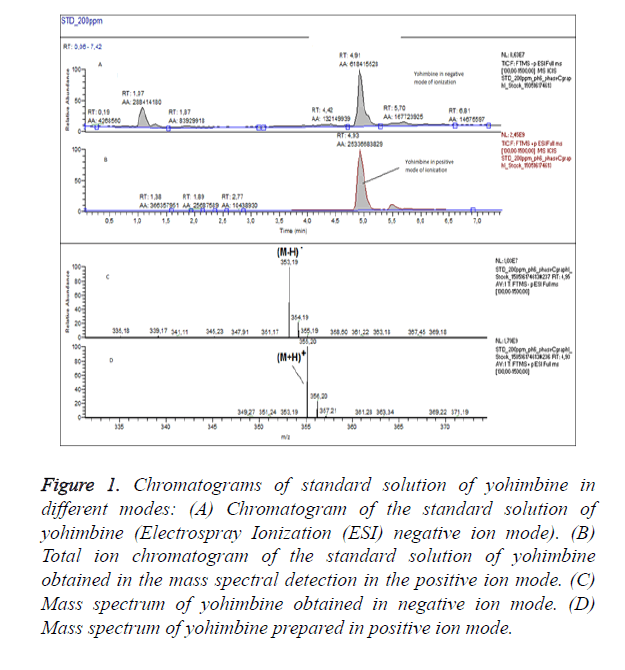
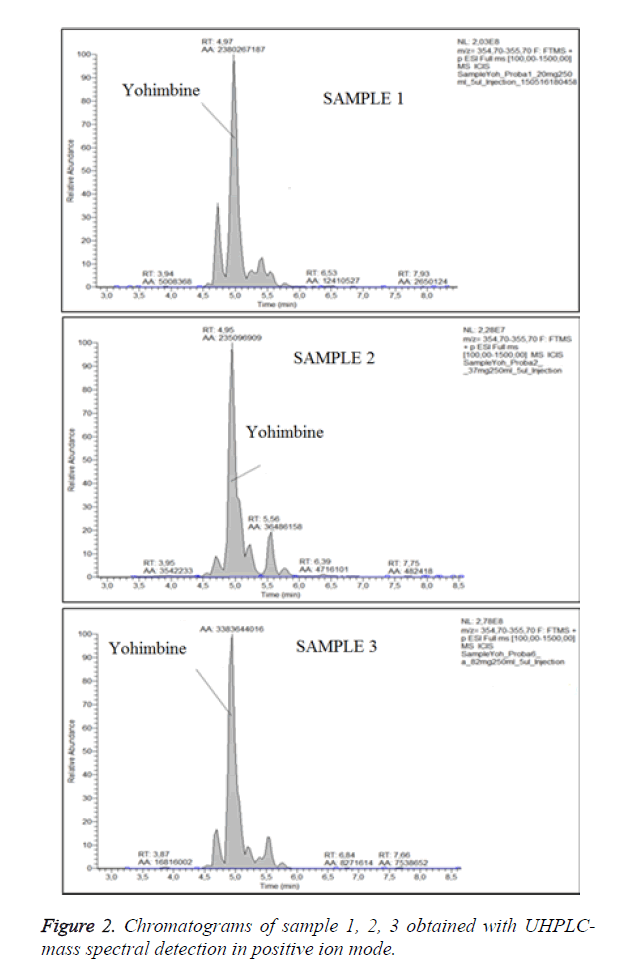
For optimization of the conditions of ionization we have obtained chromatograms of standard solution of yohimbine in positive ion mode and in negative ion mode (Figure 1). In the both modes of ionization we have observed formation of molecular ions, respectively (M-H)-with m/z 353.19 and (M +H)+ at m/z 355.20. Positive ion and negative ion mode were compared. We have found that ionization in positive mode offers a significantly higher sensitivity, with also higher selectivity. We have performed the analyses of the samples in positive ion mode with monitoring of the molecular ion of yohimbine with m/z 355.20 (Figure 2). We have found that the tree dietary supplements do not contain yohimbine in the concentration specified on the label (Table 1). These products invariably display incorrect or incomplete information on their packaging, increasing risks to consumers.
Figure 1. Chromatograms of standard solution of yohimbine in different modes: (A) Chromatogram of the standard solution of yohimbine (Electrospray Ionization (ESI) negative ion mode). (B) Total ion chromatogram of the standard solution of yohimbine obtained in the mass spectral detection in the positive ion mode. (C) Mass spectrum of yohimbine obtained in negative ion mode. (D) Mass spectrum of yohimbine prepared in positive ion mode.
| Sample | Content of yohimbine (Declared on the label) | Content of yohimbine |
|---|---|---|
| Sample 1 | 500 mg/1 capsule | 60 mg/1 capsule |
| Sample 2 | 5 mg/1 capsule | 6.1 mg/1 capsule |
| Sample 3 | 5 mg/1 capsule | 9.5 mg/1 capsule |
Table 1. Total content of yohimbine in the analysed food supplements.
We have performed a validation of the described method.
Selectivity of the method
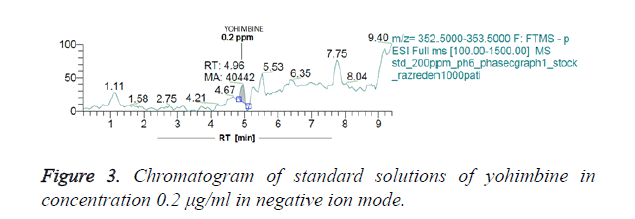
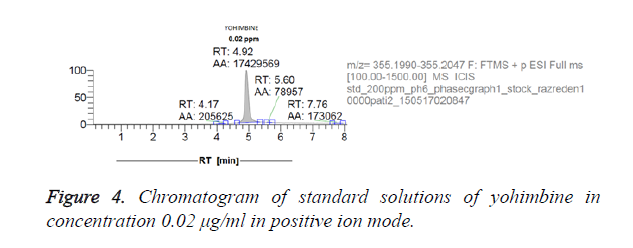
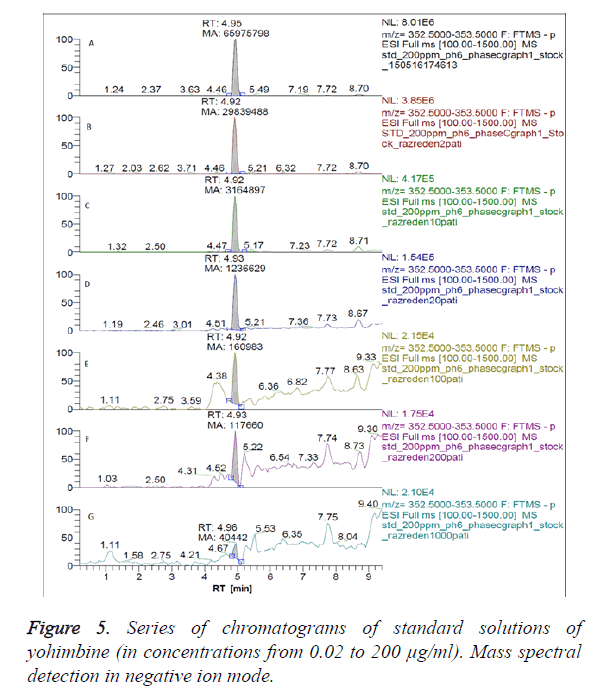
Figures 3 and 4 show chromatograms of standard solutions of yohimbine at a concentration 0.2 μg/ml and 0.02 μg/ml, respectively resulting in standby negative ionization and in positive ionization mode.
Linearity of the method
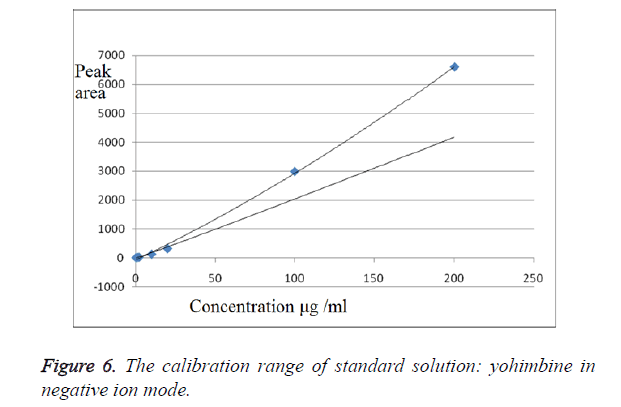
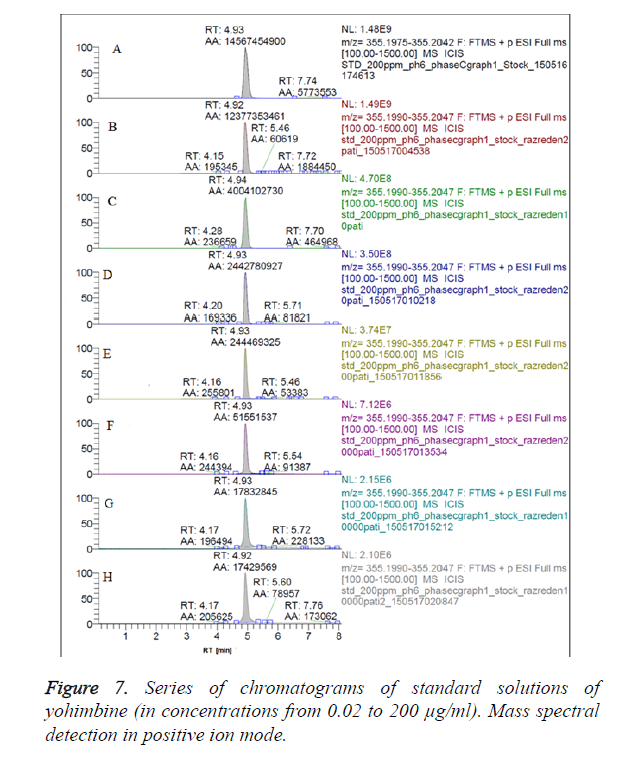
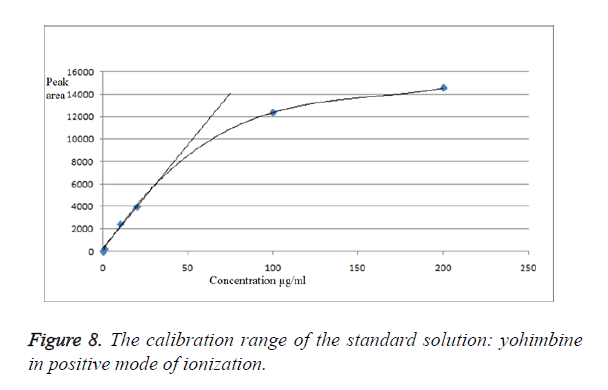
We have obtained chromatograms of a series of standard solutions of yohimbine (concentration from 0.02 to 200 μg/ml) in the monitoring of the molecular ion of yohimbine in the negative ionization mode and in the positive ionization mode (Figures 5 and 6) In both modes standard graphiques show linearity only in the concentration range up to 50 μg/ml. Figures 7 and 8 represent series chromatograms of standard solutions of yohimbine obtained with mass spectral detection in the positive ionization mode. Standard graphiques obtained with mass spectral detector show linearity in the concentration range 0-50 μg/ml.
Reproducibility was determined by introducing a 6-fold under the described chromatographic conditions and monitoring of the molecular ion of yohimbine in mode monitoring positive ions. Peak area of the chromatograms (S), the average value (͞x), standard deviation (σ) and the relative standard deviation (RS) are presented in Table 2.
| No. of the analysis | Peak area | ͞x | σ | RSD |
|---|---|---|---|---|
| 1 | 51551537 | 51280688 | 799407 | 15,58,885 |
| 2 | 49895320 | |||
| 3 | 51080734 | |||
| 4 | 51998016 | |||
| 5 | 50605314 | |||
| 6 | 51813551 |
Table 2. Reproducibility of the method.
Conclusion
The development and validation of a rapid qualitative and quantitative method based on an UHPLC/MS allowed the identification of the target compound-yohimbine in mono and multi-botanical preparations, distributed as food supplements. The described method shows high sensitivity and selectivity. We have found that in the mode of positive ion monitoring the sensitivity and selectivity are higher in comparison with detection at monitoring of negative ions. The modified method was applied for the determination of yohimbine in food supplements. The analyses showed that in some of the food supplements the real content of yohimbine is different from the declared that indicate the importance of the analytical control of food supplements.
Acknowledgment
This work is supported by Medical University Plovdiv DP 13/2015.
References
- http://tongkatali-ingredients.com/yohimbe-sources-in-west-africa.htm
- Small J, Adams F. Yohimbe bark: Its history and identification in commerce. Pharm J 1992; 108: 282-286.
- Ainslie JR. A list of plants used in native medicine in Nigeria. Imp Forest Inst 1937; 7.
- Dalziel J. The Useful plants of tropical West Africa. Supplement to: The Flora of West Tropical Africa. Kew Royal Botanic Gardens 1937.
- Oliver-Beyer B, Medicinal plants in tropical West Africa. Camb Univ Press 1986; 62.
- Tyler VE. The honest herbal-a sensible guide to the use of herbs and related remedies. Haworth Press London (3rd edn.) 1993; 327-330.
- Liu HB, Peng Y, Huang L, Xu J, Xiao PG. Mechanism of selective inhibition of yohimbine and its derivatives in adrenoceptor α2 subtypes. J Chem 2013; 2013.
- Mac CM, Mc CI, Shaw FH. The action of yohimbine on excitation and propagation in nerve. Aus J Exp Biol Med Sci 1994; 27: 115-122.
- Schum K, Pierreex B. Scientific opinion on the evaluation of the safety in use of yohimbe. EFSA J 2013; 11: 3302.
- Qinhua C, Peng L, Zhuo Z, Kaijun L, Jia L, Qiang L. Analysis of yohimbine alkaloid from Pausinystalia yohimbe by non-aqueous capillary electrophoresis and gas chromatography-mass spectrometry. J Sep Sci 2008; 31.
- Owen J, Nakatsu S, Condra M, Surridge D, Fenemore J, Morales A. Sub-nanogram analysis of yohimbine and related compounds by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1985; 342: 333-340.
- Boris Z, Karine N, Jean RI, Andrew M, Kurt H. Qualitative and quantitative determination of yohimbine in authentic yohimbe bark and 76 in commercial aphrodisiacs by HPLC-UV-API/MS methods. Phytochem Anal 2003; 14: 193-201.
- Jianghao S, Pei C. Chromatographic fingerprint analysis of yohimbe bark and related dietary supplements using UHPLC/UV/MS. J Pharm Biomed Anal 2012; 61: 42-149.
- Diquet B, Doare L, Gaudel G. New method for the determination of yohimbine in biological fluids by high-performance liquid chromatography with amperometric detection. J Chromatogr B Biomed Sci Appl 1984; 311: 449-455.