ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 20
Ultrasonic-enhanced and hot water extraction of antioxidant sulfated polysaccharides from Codium tomentosum stackhouse, 1797
Pagolu Navya and Samantha S Khora*
Department of Integrated Biology, School of Bioscience and Technology, VIT University, Vellore, Tamilnadu, India
- *Corresponding Author:
- Samantha S Khora
Department of Integrated Biology School of Bioscience and Technology VIT University Vellore Tamilnadu India
Accepted date: August 29, 2017
Sulfated polysaccharides were isolated from green seaweed Codium tomentosum by using two different extraction processes: Hot water extraction (HEP) and Ultrasound Enhanced Extraction (UEP). The yield, chemical composition and antioxidant activity of isolated polysaccharides were determined and compared. In present study, the ultrasound-enhanced extraction method influenced a usual yield and extraction time. It increases the yield up to 16.2% in 45 min from 12.35% in 5 h of hot water extraction yield. The chemical composition of UEP was also significantly affected and showed higher sulfate and uronic acids content. Both samples presented comparable good antioxidant activities on hydrogen peroxide, nitric oxide, ABTS, DPPH, deoxyribose radicals. However the UEP exhibited higher antioxidant potential than HEP. The IR spectra of both sulfated polysaccharides revealed characteristic absorption bands. It shows the presence of hydroxyl, carboxyl and sulfate groups. This study revealed that chemical composition and antioxidant activity of polysaccharides obtained from Codium tomentosum vary according to the process for their extraction. Moreover, sulfated polysaccharides obtained by UEP or HEP both can be a good source of natural antioxidants and can be harnessed for further biotechnological applications.
Keywords
Codium tomentosum, Ultrasonication, Sulfated polysaccharides, Antioxidant activities.
Introduction
Marine organisms especially marine algae have an abundant source of various bioactive compounds with provide beneficial pharmaceutical potentials [1]. They are found to be a reliable source of healthy food because they are found to have low content in lipids, high concentration in polysaccharides, polyunsaturated fatty acids, and abundance in minerals, vitamins and also due to their content in bioactive molecules. Among macromolecules, polysaccharides have the maximum capacity for transmitting biological information due to their increased potential for structural variability [2,3]. The polysaccharides of marine origin offer a safer activity than mammalian polysaccharides for drug discovery. The most abundant and extensively studied polysaccharides are the sulfated polysaccharides which are obtained from non-animal origin [4].
Codium species have been utilized as food for cultured abalone, they are consumed by humans and they are also a varied source of bioactive compounds [5]. Metabolites of this group of species, in general, have been known to exhibit interesting biological activities e.g. antibacterial, antiviral, antiinflammatory, antioxidant and cytotoxic and ichthyotoxic activities etc. [6]. There is no study concerning sulfated polysaccharides of these species (Codium tomentosum) which is available in the Indian coasts and the narrow number of studies on this species, which are obtained from other sites, have focused on its fatty acid composition, the antioxidant, and anti-genotoxic potential of a crude ethanolic extract, antitumourigenic, hypoglycemic activities, anti-arthritic, amylase activities of crude extracts [7-11].
Extraction of bio-compounds from plants is usually performed by conventional methods. It involves consumption of more amounts of solvent and an increase in the extraction time [12]. Although, other techniques, including supercritical carbon dioxide extraction, subcritical water extraction, microwave extraction and ultrasound extraction have increased significant alternatives to the conventional methods [13]. Among these, the Ultrasound Extraction (UE) method provides important advantages for the extraction process of organic compounds from plants using a solvent. Previous studies described that UE technology can minimize the extraction time and solvent use [14]. Currently, to extract active compounds such as rutin and querceting sonication have been utilized, antioxidants, polysaccharides and bioactive principles from plant materials. However, there are fewer evidences on ultrasound-enhanced extraction of sulfated polysaccharides from seaweeds [15-20].
The algal polysaccharides were also reported to play a vital role as free radical scavengers by preventing and repairing damages caused by reactive oxygen species [19,20]. However, to our best of knowledge, no information is available regarding the impact of ultrasound-assisted extraction method on biological properties of seaweed polysaccharides which is a point of interest in our study. Hence, the present study was focused on the investigation of antioxidant activity of Codium tomentosum containing sulfated polysaccharides using both ultrasound-enhanced and hot water extraction methods.
Materials and Methods
Seaweed and extraction of sulfated polysaccharides
The fresh specimens of green seaweed Codium tomentosum were collected along the coast of Tamil Nadu, India, in August, 2015. The seaweed species was identified and authenticated by Ramalingam, Regional Centre of Central Marine Fisheries Research Institute (CMFRI), Mandapam, Tamilnadu, India. The collected alga is first washed in seawater to eliminate the macroscopic epiphytes, and then washed thoroughly with distilled water to remove dust particles. The specimen was shade dried and grounded into powdered.
Hot water extraction of polysaccharides was performed by the procedure previously described by Subash et al. with little modification [21]. Briefly 100 g of algae powder was then mixed with the distilled water at a mass/volume ratio of 1:10 and kept at room temperature for overnight, then homogenized and refluxed at 90-95°C for 4 h. The syrup found to be brown in color was then filtered through Whatman No. 3 filter paper, which was then concentrated to 1/4th of the original volume, cooled and allowed to be precipitated with three volumes of ethanol. The obtained precipitate was collected by centrifugation and lyophilized.
Ultrasound-enhanced extraction of polysaccharides was performed by the method as described by Shao et al. with little modifications [19]. 100 g of algae powder was mixed with distilled water at a mass/volume ratio of 1:10 and kept at room temperature for overnight. The Sonics vibra cell (VCX500), input ultra-sound power was 500W, 20 KHz was used. The extraction time and temperature was 30 min and 45°C respectively. After cooling the slurry was filtered and concentrated to 1/4th of the original volume, cooled and then precipitated with three volumes of ethanol. The obtained precipitate was collected by centrifugation and lyophilized.
The HEP and UEP were further partially purified by column chromatography. The crude polysaccharides were dissolved in distilled water (10 μg/10 ml) centrifuged at 6000 rpm for 10 min and the supernatant was applied to a DEAE-cellulose column (3 × 45 cm) equilibrated with distilled water. The column was eluted step-by-step using distilled water by a linear gradient of 0-3 mol/NaCl at a flow rate of 0.55 ml/min and 15 fractions were collected. These fractions were estimated for the carbohydrate content by phenol-sulphuric acid method as described by Dubois et al. [22]. The fractions showing higher content were pooled together dialyzed for 24 h against distilled water and then they were lyophilized. The active fractions were identified by carbohydrate estimation using phenol-sulphuric acid method and it was utilized for the further study.
Chemical analysis
The total carbohydrates content was estimated by phenol sulphuric acid method as reported by Dubois et al. [22]. The sulfate content was estimated by the barium chloride gelatin method of Lloyd et al. [23]. Estimation of uronic acid content by the carbazole-sulfuric acid method as described previously by Egan et al. [24].
Analysis of elements
The percentage content of carbon, hydrogen, nitrogen and sulfur of isolated polysaccharides were assessed by the operating mode of PE 2400 series II CHNS/O analyzer EA1112 (CE Instrument, Italy). Approximately 1 mg of sample was injected, and then eluted with TCD (Thermal conductivity detector, CE Instrument). 2, 5-Bis (5′ tert-butyl-2- benzoxazol-2-yl) thiophene (BBOT) and aspartic acid were utilized as reference standard.
FT-IR spectrophotometer analysis
Infrared spectra of polysaccharides were tested using Perkin- Elmer FT-IR instrument which helped to evaluate different sulfate, carboxyl and hydroxyl groups present in these sample molecules [25]. One part of extract was then mixed along with ninety nine parts of dried potassium bromide (KBr) separately and then compressed to formulate a salt disc of 3 mm diameter. These discs were subjected to IR- spectrophotometer, and then read the absorption between 400 and 4000 cm-1.
In Vitro Antioxidant Activity
Determination of reducing power
Reducing power of the polysaccharides was estimated by the following method of Yamaguchi et al. [26]. In this estimation, 4 ml of the reaction mixture, which contains samples of different concentration in phosphate buffer (0.2 M, pH 6.6) was incubated for 20 min with potassium ferricyanide (1% w/v) at 50°C. The reaction was terminated by the addition of TCA solution (10% w/v). The solution was then mixed along with distilled water and ferric chloride (0.1% w/v) solution and the absorbance was measured at 700 nm.
Hydrogen peroxide scavenging assay
The hydrogen peroxide radical scavenging activity was estimated by hydrogen peroxide assay as described by Gulcin et al. [27]. Hydrogen peroxide (10 mM) solution was made in phosphate buffered saline (0.1M, pH 7.4). 1ml of the polysaccharides of different concentration (100, 250, 500, 750 and 1000 μg) was swiftly mixed with 2 ml of hydrogen peroxide solution. After 10 min of incubation, the absorbance was measured at 230 nm in the UV spectrophotometer at 37°C against a blank (without hydrogen peroxide). The percentage of scavenging of hydrogen peroxide was calculated by the formula:
Percentage scavenging (H2O2)=((Ao-A1)/Ao) × 100
Ao-Absorbance of control; A1-Absorbance of sample.
DPPH radical scavenging assay
The DPPH radical scavenging activity of polysaccharides was measured by the 1-1-Diphenyl-2-picryl-hydrazyl (DPPH) following the method of Blois [28]. DPPH was utilized as a reagent which is a more accurate and convenient method for titrating the oxidizable groups of synthetic (or) natural antioxidants. 0.1 mM solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of polysaccharides of different concentration (100, 250, 500, 750 and 1000 μg). After 10 min, absorbance was read at 517 nm. The percentage scavenging activity values were calculated by the following formula:
Percentage of Scavenging=((Ao-A1)/Ao) × 100
Where Ao is absorbance of control and A1 is absorbance of sample turbidity factor.
ABTS inhibition assay
The ability of the polysaccharides to scavenge ABTS radical was determined by the method of Re et al. [29]. ABTS (2, 2 azino bis (3-etheylbenzothiazoline-6-sulphonicacid) diammonium salt) was produced by mixing 5 ml of 7 mM ABTS with 88 μl of 140 mM potassium per sulfate under darkness at room temperature for 16 h. The solution was then diluted with 50% ethanol and the absorbance at 734 nm was measured. The ABTS radical cation scavenging activity was assessed by mixing 5 ml ABTS solution (absorbance of 0.7 ± 0.05) with 0.1 ml of polysaccharide (100, 250, 500, 750 and 1000 μg). The absorbance was measured at 743 nm with spectrophotometer. The percentage of scavenging was calculated by using the formula:
% of scavenging=((Ao-A1)/Ao) × 100
Where Ao-Absorbance of control; A1-Absorbance of sample.
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was evaluated by studying the competition between deoxyribose and test compounds for hydroxyl radical produced by Fe3+- Ascorbate EDTA H2O2 system, based on the method of Kunchandy and Rao [30]. The hydroxyl radicals attack deoxyribose that finally resulted in TBARS formation. The reaction mixture contained limited to a final volume of 1.0 ml, 100 μl of 2-deoxy-2-ribose (28 mM in potassium phosphate-potassium hydroxide buffer, pH 7.4) 500 μl solutions of various concentrations of polysaccharide (100, 250, 500, 750 and 1000 μg) and standard in KH2PO4-KOH buffer (20 mM, pH 7.4), 200 μl of 1.04 mM ethylene diamine tetra acetic acid and 200 μl of 200 μM ferric chloride, 100 μl of 10 mM hydrogen peroxide and 100 μl of 1.0 mM ascorbic acid was incubated at 37°C for 1 h.
The free radical damage made on the substrate, deoxyribose (TBARS) was determined by the method of Yuan and Walsh, 1.0 ml of thiobarbituric acid (TBA) (1%) and 1.0 ml of trichloroacetic acid (2.8%) (TCA) were added and incubated at 100°C for 30 min [31]. After it becomes cooled, the absorbance was then measured at 535 nm against control containing deoxyribose and buffer. The percentage scavenging was calculated by the comparing the result of the test compound and control using following formula:
Radical scavenging activity (%)=(A0-A1/A0) × 100
(Where A0-Absorbance of control; A1-Absorbance of sample).
Superoxide anion radical scavenging assay
Superoxide anion scavenging activity was measured based on the method of Nishimiki et al. [32]. 1 ml of nitro blue tetrazolium (NBT) solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 ml of NADH solution (468 μM in 100 mM phosphate buffer, pH 7.4) and 0.1 ml of the sample at various concentrations (100, 250, 500, 750 and 1000 μg) were mixed and the reaction was initiated by adding 100 μl of phenazine methosulphate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH (7.4). The reaction mixture was incubated at 25°C for 5 min and the absorbance was read at 560 nm against blank samples. The percentage scavenging value was calculated as follows:
Radical scavenging activity (%)=(A0-A1/A0) × 100
(Where A0-Absorbance of control; A1-Absorbance of sample)
Nitric oxide radical scavenging assay
Nitric oxide radicals produced from sodium nitroprusside solution at physiological pH reacts with oxygen to produce nitrite ions which were estimated by the Griess reaction [27]. 2 ml of sodium nitroprusside (10 mM) was mixed along with 1 ml of the polysaccharide with different concentrations (100, 250, 500, 750 and 1000 μg) in phosphate buffer (pH 7.4). The mixture was incubated at 25°C for 150 min. 1 ml sulphanilic acid reagent (0.33% sulphanilamide in 20% acetic acid) was added to 0.5 ml of the incubated solution and allowed for 5 min for completing diazotization. Then, 1 ml of 0.1% napthyl ethylene diamine dihydrochloride was added and incubated for about 30 min at room temperature. Absorbance was taken at 540 nm and percentage scavenging was calculated as follows:
Radical scavenging activity (%)=(A0-A1/A0) × 100
(Where A0-Absorbance of control; A1-Absorbance of sample).
Statistical analysis
All of the data were expressed as means ± standard deviation (SD) of three replications, and the ANOVA test was used for statistical analysis. The values were considered to be significantly different when the p value was less than 0.05.
Results and Discussion
Several studies detailed the sulfated polysaccharides, particularly in seaweeds contain large amounts, when compare to terrestrial plants and trees. In the present study, ultrasoundenhanced extraction is influenced a usual yield; it increases the yield up to 16.2% from 12.35% of hot water extraction yield. Simultaneously the total extraction time was also shortened. He et al. also used hot water for the obtaining the extract of A [33]. Subcrenata lischke which was precipitated using 80% ethanol and obtained only 9.32 g of crude polysaccharides. Wang et al., employed first time ultrasound technology to obtain arabinoxylan from wheat bran and the experimental yield was found to be 142.6 ± 0.17 mg/g, which is equivalent with the predictive yield and he suggested ultrasound increased the state of enzymatic treatment with higher extraction yield [34]. Several authors also have cited the utilization of sonication to increase the extraction of polysaccharides in plants [35,36]. So, ultrasonic extraction method can enhance shortening the extraction time, extraction efficiency and reducing the consumed energy. Along with this, the main advantage of acquiring intact thermo-labile polymers would be achievable. The polysaccharides were isolated and purified by ethanol precipitation and then using DEAE-cellulose ion exchange chromatography. The utilization of DEAE-cellulose as a matrix has been largely reported for the separation of polysaccharide and also to uncover the characteristics of different species of algae, such as on Ecklonia cava, Champia feldmannii [37,38].
UEP shows relatively higher sulfate, uronic acid content than HEP and also compared to other studies on sulfated polysaccharide [39,40]. The percentage of total carbohydrate of UEP 65.84 ± 0.27% was higher than HEP 59.13 ± 0.31%. Specifically, the sulfate content in UEP 16.38 ± 0.81% is much greater than in HEP 10.18 ± 0.24%. The antioxidant properties of carrageenans appeared related to sulfate content [41]. The Uronic acid content in UEP 8.31 ± 0.72% was more than HEP 5.99 ± 0.18%. Uronic acid also plays a vital role on the bioactivities. Generally many polysaccharides are acid complex carbohydrate, which are composed of uronic acid.
Elemental analysis of UEP and HEP were analyzed. The percentage of elemental carbon, hydrogen, nitrogen and sulfur content are depicted in Table 1.
| Polysaccharides | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Sulfur (%) |
|---|---|---|---|---|
| UEP | 30.45 | 9.12 | 6.24 | 12.89 |
| HEP | 23.63 | 4.96 | 3.54 | 8.74 |
Table 1: Elemental analysis of sulfated polysaccharides from Codium tomentosum.
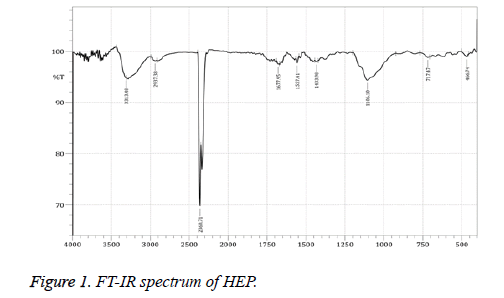
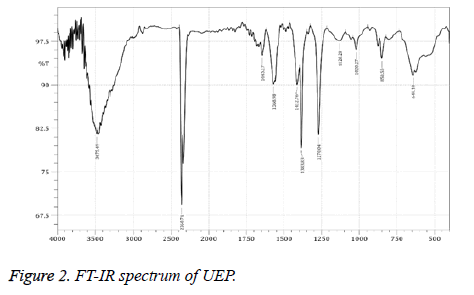
FT-IR Spectrophotometer analysis
The FT-IR spectrum HEP is shown in Figure 1. The strong peak at 3313.48 cm-1 explained O-H stretching vibration (carboxylic acid group) even the signal at 2937.38 and 2360.71 cm-1 cleared the C-H stretching vibration (alkanes) [42]. The signal at 1677.95 and 1557.41 cm-1 contributed C=O medium stretching vibration (carbonyls). The band 1433.98 and 1106.1 cm-1 corresponding to C-C stretch (aromatic) and C-N stretch (aliphatic amines) bending is plane. The week signals 466.74 cm-1 corresponding to ring formation. The FT-IR spectrum of UEP is cited in Figure 2. The prominent peak at 3475.49 cm-1 explained O-H stretching vibration (alkyl group) even the signal at 2360.71 and 1645.17 cm-1 cleared the C-H stretching vibration [43]. The signal at 1620.21 and 1568.98 cm-1 contributed N-H bend stretching vibration. The band 1412.76 and 1383.83 cm-1 corresponding to OH bending is plane. The signal at 1270.04 and 1128.28 cm-1 is C-H wag (-CH2X). The signal at 850 to 644 indicated sulfate groups [44].
Antioxidant activity
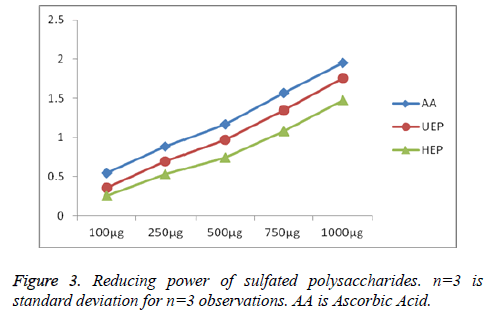
The reducing properties are usually associated with the presence of reductant, which have been shown to produce antioxidant action by splitting the free radical chain by donating a hydrogen atom [45]. The reducing power of UEP and HEP was measured, and results demonstrated that they had noticeable effect shown in Figure 3. The reducing power of polysaccharides from UEP (0.354 ± 0.022%)-(1.755 ± 0.014%), is higher than HEP (0.256 ± 0.017%)-(1.449 ± 0.027%) and were compared with the standard ascorbic acid (AA) (0.547 ± 0.025%)-(1.953 ± 0.015%); might be due to higher sulfate content. Referring to the report of Huimin et al. that high sulfate content ulvans had more effective reducing power, scavenging activity on hydroxyl radical, chelating ability on ferrous ion, than natural ulvan [46].
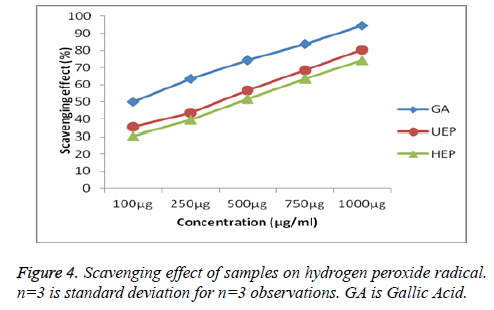
Many species of seaweed endowed scavenging ability of hydrogen. It move across membranes and may gradually oxidize a number of compounds peroxide [47]. In this work the scavenging effect of both samples on hydrogen peroxide radicals. The maximum scavenging activity was shown by UEP 80.24 ± 0.11% and HEP also shows 74.37 ± 0.22% of inhibition. The hydrogen peroxide scavenging effect of samples were compared with the standard Gallic acid (GA) is cited in Figure 4.
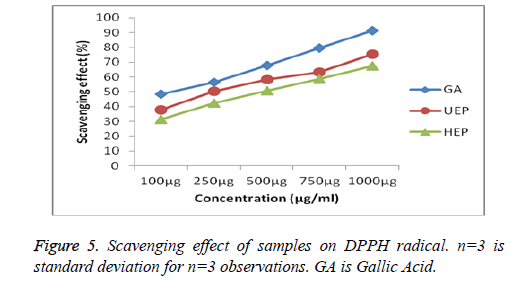
A DPPH solution which is freshly prepared shows a deep purple color. This purple color usually fades/disappears when an antioxidant is present in the medium and which results in a decrease in absorbance at 517 nm. Hence, the more quickly the absorbance decreases, the more potent is the antioxidant activity of the substance. The DPPH radical scavenging effect of UEP and HEP were studied and compared with the standard Gallic acid presented in Figure 5. The scavenging effect increased with concentration of standards and samples. The DPPH scavenging activity for the UEP shows 75.52 ± 0.294% relatively higher than HEP 67.49 ± 0.239%. DPPH have been used widely as a free radical to evaluate reducing substances [48]. ABTS assay is a simple indirect assay for determining the activity of natural antioxidants. ABTS radical is stable, in the absence of phenolics, but in the presence of an H-atom donor such as phenolics it reacts energetically, being converted into a non-colored form of ABTS [49].
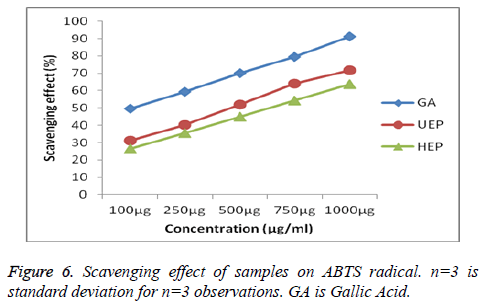
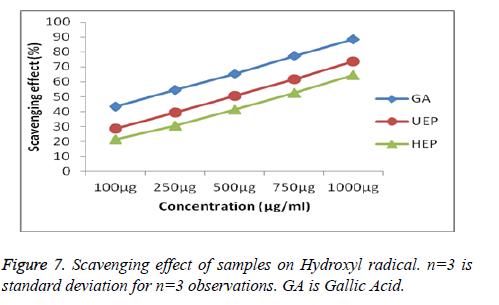
Scavenging activities UEP and HEP on ABTS radical were also measured and compared with the standard Gallic acid presented in Figures 6 and 7. As the figure showed, all the samples had substantial scavenging effects with dose-effect relationship and the effects were for the UEP is 71.62 ± 0.19% shows good scavenging activity than HEP 63.61 ± 0.24%. Hydroxyl radicals are recognized to be the most reactive among all the reduced forms of di oxygen and are known to initiate cell damage in vivo [50]. Further, the effect of UEP and HEP on hydroxyl radicals produced by Fe3+ ions was measured by the extent of deoxyribose degradation, by reactive OHgenerated from Fenton’s type reaction and compared with the standard Gallic acid shown in Figure 8. The hydroxyl scavenging effect of UEP is 73.59 ± 0.22% and HEP shows comparably less percentage of inhibition 64.54 ± 0.31%.
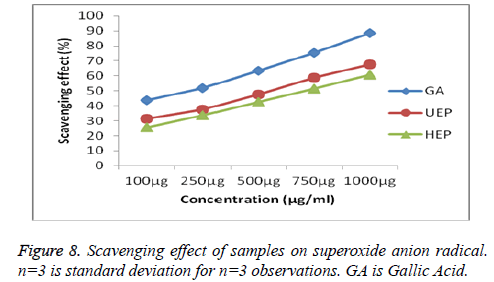
Superoxide radicals were produced in a PMS/NADH system utilized to be assayed in the reduction of NBT [26]. Scavenging activities of UEP and HEP on superoxide anion were also studied and compared with the standard Gallic acid presented in Figure 8. As the figure showed, all the samples were found to have significant scavenging effects with doseeffect relationship and the effects were for the UEP is 67.53 ± 0.31% and HEP shows 60.56 ± 0.33% inhibition.
NO is a diffusible free radical, which is involved in many roles as an effectors molecule in various biological systems including neuronal messenger, vasodilation, antimicrobial and antitumor activities [51]. Scavenging activities of the two samples (UEP and HEP) on nitric oxide also had noticeable effect shown in Figure 9. The nitric oxide scavenging effect for the UEP is 66.37 ± 0.28% and HEP shows 55.54 ± 0.29% of inhibition. Chen et al., reported that the scavenging effects of the free radicals increased with an increased in the content of uronic acid in different tea polysaccharide conjugate fractions, the presence of uronic acid might show effect on the physicochemical properties of the polysaccharides and hence their bioactivities [52,53]. As supported above study the present results indicated that the content of sulfate and uronic acid in UEP was much higher and also shows greater antioxidant ability when compared to HEP, probably correlating with their chemical composition and extraction conditions.
Conclusion
Antioxidant activities of sulfated polysaccharides isolated from seaweeds have been studied by many researches and also little information is available on their chemical composition and biological activities. However polysaccharides obtained by different extraction methods seem to be imperative for the utilization of these kinds of marine resources. In the present study, when compared to HEP, the UEP showed higher in yields, chemical composition and they were also shown good scavenging ability on hydrogen peroxide, nitric oxide, ABTS, DPPH, deoxyribose radicals. These results clearly indicate the beneficial effects of algal polysaccharides. It can be concluded that the mechanical action developing from ultrasound waves under the extraction time and temperature conditions used, promote the release of extra polysaccharides from the intracellular or wall contents of seaweed. These preparations constitute novel polysaccharide based antioxidants, potentially applicable in food industry, cosmetics, pharmacy and further investigation is needed to explore its bioactivities.
Acknowledgment
This work was carried out with support of VIT University for providing the facilities to conduct this part of research work.
References
- Glaser B, Alejandro MSM. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality Keith. Biochem Pharmacol 2009; 78: 440-448.
- Barrow C. Shahidi F. Marine nutraceuticals and functional foods. CRC Press, New York 2008.
- Karim S, Jessica P, Farida G, Christine DL, Corinne S, Je Ratiskol, Gaston Godeau, Anne-Marie Fischer, Dominique Helley, and Sylvia Colliec-Jouault. Marine Polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar Drugs 2011; 9: 1664-1681.
- Pereira MS, Silva ACESV, Valente AP, Mourao PAS. A 2-sulfated, 3-linked -l-galactan is an anticoagulant polysaccharide. Carb Res 2002; 337: 2231-2238.
- Verbruggen H, Leliaert F, Maggs CA, Shimada S, Schils T, Provan J, Booth D, Murphy S, De Clerck O, Littler DS, Littler MM. Coppejans E. Species boundaries and phylogenetic relationships within the green algal genus Codium (Bryopsidales) based on plastid DNA sequences. Mol Phylogenet Evol 2007; 44: 240-254.
- Siddhanta AK, Shanmugam M. Metabolites of tropical marine algae of the family Codiaceae (Chlorophyta): Chemistry and bioactivity. J Indian Chem Soc 1999; 76: 323-334.
- Khotimchenko SV. Fatty acids of the species in the genus Codium. Botanica Marina 2003; 46: 456-460.
- Celikler S, Ozgur V, Gamze Y, Rahmi B. Evaluation of anti-oxidative, genotoxic and antigenotoxic potency of Codium tomentosum Stackhouse ethanolic extract in human lymphocytes in vitro. Food Chem Toxicol 2009; 47: 796-801.
- El-Masry MH, Mostafa MH, Ibrahim AM, El-Naggar MMA. Marine algae that display anti-tumorigenic activity against Agrobacterium tumefaciens. FEMS Microbiol Lett 1995; 128: 151-155.
- Lamela M, Anca J, Villar R, Otero J, Calleja JM. Hypoglycemic activity of several seaweed extracts. J Ethnopharmacol 1989; 27: 35-43.
- Aparanji P, Atika R, Venu GS, Rajan P. Evaluation of Anti-Arthritic, Antimicrobial and Amylase activities of Codium tomentosum from Andaman and Nicobar islands. Int J Curr Microbiol App Sci 2013; 2: 255-266.
- Yan X, Suzuki M., Ohnishi-Kameyama M, Sada Y, Nakanishi T, Nagata T. Extraction and identification of antioxidants in the roots of yacon (Smallanthus sonchifolius). J Agric Food Chem 1999; 47: 4711-4713.
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 2006; 17: 300-312.
- Mason TJ, Cordemans ED. Ultrasonic intensification of chemical processing and related operations: a review. Trans Inst Chem Eng 1996; 974: 511-516.
- Yang Y, Zhang F. Ultrasound-assisted extraction of rutin and quercetin from Euonymus alatus (Thunb). Sieb Ultrason Sonochem 2008; 15: 308-313.
- Albu S, Joyce E, Paniwnyk L, Lorimer JP, Mason TJ. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason Sonochem 2004; 11: 261-265.
- Yang X, Zhao Y, Zhou Y, Lv Y, Mao J, Zhao P. Component and antioxidant properties of polysaccharide fractions isolated from Angelica sinensis (OLIV.) DIELS. Biol Pharm Bull 2007; 30: 1884-1890.
- Vinatoru M, Toma M, Radu O, Filip PI, Lazurca D, Mason TJ. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason Sonochem 1997; 4: 135-139.
- Shao P, Xiaoxiao C, Peilong S. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int J Biol Macromol 2013; 62: 155-161.
- Wan P, Yang X, Cai B, Chen H, Sun H, Chen D, Pan J. Ultrasonic extraction of polysaccharides from Laminaria japonica and their anti-oxidative and glycosidase inhibitory activities. J Ocean Univ 2015; 14: 651-662.
- Subash A, Hanumantha R, Balaji R, Adoor GS, Veeraraghavan G, Ganapathy R, Hannah RV. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornate (Marine Brown Alga). Food Chem Toxicol 2010; 48: 87-192.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956; 28: 350-356.
- Lloyd AG, Dodgson KS, Price RG, Rose FAI. Polysaccharide sulphates. Biochimica et Biophysica Acta 1961; 1: 108-115.
- Egan H, Kirk RS, Sawyer R. Pearson?s Chemical Analyses of Foods (8th Ed). London, UK 1981.
- Wang Y, Zhang M, Ruan D, Shaskov AS, Kilcoyrs M, Savage AV, Zhang L. Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos. Carbohydr Res 2004; 339: 327-334.
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical ? scavenging activity of foods by using 1, 1-Diphenyl-2-Picryl hydrozl. Biosci Biotechnol Biochem 1998; 62: 1201-1204.
- Gulcin T, Irfan KO, Kufrevioglu M, Oktay M, Buyukokuroglu ME. Antioxidant, antimicrobial, antiuclcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 2004; 90: 205-215.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-1200.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Evans C. Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Radic Biol Med 1999; 26: 1231-1237.
- Kunchandy R. Antioxidant properties of dried kayamo-nori, a brown alga Scytosiphon lomentaria. Food Chem 1990; 89: 617-622.
- Yuan YV, Walsh NA. Antioxidant and anti-proliferative activities of extracts from a variety of edible seaweeds. Food and Chem Toxicol 2006; 44: 1144-1150.
- Nishimiki M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972; 46: 849-853.
- He YM, Liu CH, Chen YX, Ji AC, Shen ZL, Xi T, Yao QS. Isolation and structural characterization of a novel polysaccharide prepared from Arca subcrenata Lischke. J Biosci Bioeng 2007; 104: 111-116.
- Wang J, Sun, B, Cao, Y, Tian, Y, Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem 2008; 106: 804-810.
- Hromádková Z, Ko?t?álová Z, Ebringerová A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason Sonochem 2008; 15: 1062-1068.
- Silveira M, Fidelis RF, Costa GP, Telles MSSP, Dantas-Santos CB, Oliveira. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int J Mol Sci 2012; 13: 409-426.
- Athukorala Y, Jung WK, Vasanthan T, Jeon YJ. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym 2006; 66: 184-191.
- Assreuy AMS, Gomes DM, Silva MSJ, Torres VM, Siqueira RCL, Pires AF. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biol And Pharm Bull 2008; 3: 691-695.
- Mahendran S, Saravanan S. Purification and in vitro antioxidant activity of polysaccharide isolated from green seaweed Caulerpa racemosa. Int J Pharm Bio Sci 2013; 4: 1214-1227.
- Gabriel PF, Rafael BGC, Moacir FQ, Mariana Santana Santos PS, Pablo CS, Hugo AOR, Leandro SC. Proteolysis, NaOH and Ultrasound-Enhanced Extraction of anticoagulant and Antioxidant Sulfated Polysaccharides from the Edible Seaweed, Gracilaria birdiae. Mol 2014; 19: 18511-18526.
- Rocha de Souza MCR, Marques CT, Dore CMG, Ferreira da Silva FR, Rocha HAO, Leite EL. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J Appl Phycol 2007; 19: 153-160.
- Parthiban K, Vignesh V, Thirimurugan R. Charecterisation and in vitro studied on anticancer activities of exopolymaer of Bacillus thureinginesis S13. Afr J Biotechnol 2014; 13: 2137-2144.
- Andreas B. Infrared spectroscopy of proteins. Biochimica et Biophysica Acta. 2007; 1767: 1073-1101.
- Abd E, Baky H, Hanaa E, Baz KF, Latife SA. Induction of sulfated polysaccharides in Spirulina platensis as response to nitrogen concentration and its biological evaluation. J Aquac Res Development 2013; 5: 1-8.
- Wang J, Zhang Q, Zhang Z, Li. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol 2008; 42: 127-132.
- Huimin QI, Quanbin Z, Tingting Z, Zhien H. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Bio Macromol 2006; 37: 195-199.
- Gordon MH. The mechanism of antioxidant action in vitro. In: BJF Hudson, Editor. Food Antioxidants Elsevier Applied Science, London 1990; 1-18.
- Siriwardhana N, Lee KW, Kim SH, Ha JW, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Tech Int 2003; 9: 339-346.
- Cotelle N, Bemier, JL, Catteau JP, Pommery J, Wallet JC, Gaydou, EM. Antioxidant properties of hydroxyl-flavones. Free Radical Biol Med 1996; 20: 35-43.
- Roginsky V, Lissi EA. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 2005; 92: 235-254.
- Rollet LE, Grange MJ, Elbim C, Marquetty C, Gougerot-Pocidalo MA, Pasquier C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic Biol Med 1998; 24: 563-572.
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem 1998; 46: 1887-1892.
- Chen H, Zhang M, Xie B. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J Agric Food Chem 2004; 52: 3333-3336.