ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 6
Up-regulation of various cytokines in pulp tissues after experimental tooth movement in rats
1Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University Graduate School of Biomedical Sciences, 1-2-3 Kasumi, Minami-ku, Hiroshima, Japan
2Department of Orthodontics, Kanagawa Dental University, Ineoka-cho, Yokosuka-city Kanagawa, Japan
- *Corresponding Author:
- Masato Kaku
Department of Orthodontics and Craniofacial Developmental Biology
Hiroshima University Graduate School of Biomedical Sciences, Japan
Accepted date: November 7, 2016
Aim: Previous studies have demonstrated that orthodontic forces may injure dental pulp tissue via the apical foramen. The purpose of this study was to investigate the expression of various cytokines in pulp tissues of root apex after orthodontic tooth movement and compare the amount of internal root resorption in vital and pulp less rat teeth.
Methods: Upper right first molars of 7-week Wistar rats were subjected to pulpectomy and root canal filling. Both upper first molars were moved medially for 28 days (10 and 50 g force). The amount of internal root resorption and number of TRAP positive cells around the root apex were determined and expression of Interleukin-1β (IL-1β), Tumor Necrosis Factor-α (TNF-α), Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) and Macrophage Colony-Stimulating Factor (M-CSF) in pulp tissue of root apex was examined by immunohistochemistry.
Results: The expression of these factors in 50 g force groups was significantly higher in vital tooth than in the 10 g groups. However, expression of these factors was not detected in the control group. The number of odontoclasts around root apex in vital teeth was significantly more than in the pulpectomized teeth (p<0.05). The number of odontoclasts and the amount of internal root resorption in vital teeth were significantly more than in pulpectomized teeth (p<0.05).
Conclusions: This study demonstrates that during orthodontic tooth movement, injured pulp cells express these bones resorbing cytokines; as a result, internal apical root resorption may occur because of derived odontoclasts.
Keywords
Orthodontic tooth movement, Pulp tissue, Root resorption, Interleukin-1β, Tumor necrosis factor-α, Receptor activator of nuclear factor kappa-B ligand, Macrophage colony-stimulating factor.
Introduction
Orthodontic treatment is necessary for improvement of malocclusion; however, it may also cause root resorption which is a serious clinical problem. Massler et al. found that root resorption occurred in 86.4 per cent of orthodontic patients [1]. In addition, Weltman et al. reported that after orthodontic treatment, severe root resorption was observed in 1-5 per cent of incisors [2]. According to Marques et al. the incidence of severe External Apical Root Resorption (EARR) of the incisors after orthodontic treatment was 14.5 per cent [3]. Root resorption associated with orthodontic treatment is related to many factors such as orthodontic force [4-6], orthodontic period [7-10], patient’s gender [5], patient’s age [4,11-13], root shape [7,13,14], and overjet and overbite [5]. However, the essential cause of severe root resorption has not been clearly solved yet. Orthodontic root resorption progresses with cell reactions by odontoclasts, which are similar to osteoclasts in morphology, activity, functions and features [15]. Osteoclastogenesis is mainly regulated by two cytokines, Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) and Macrophage Colony Stimulating Factor (MCSF) [16,17]. RANKL expressed in the plasma membranes of osteoblasts/stromal cells is a member of the membraneassociated Tumor Necrosis Factor (TNF) ligand family and induced osteoclast formation from osteoclast progenitors [18,19]. M-CSF is also essential for the survival and proliferation of osteoclast progenitors. M-CSF is a secreted cytokine that directs hematopoietic progenitor cells along the macrophage and osteoclast lineage by binding to its receptor cfms (CD115). M-CSF is produced by monocytes, endothelial cells, and granulocytes where its production is stimulated by IL-1, Platelet Derived Growth Factor (PDGF), Interferon gamma (IFNγ) and Granulocyte Macrophage Colony- Stimulating Factor (GM-CSF) [20]. Thus, M-CSF and RANKL are considered indispensable to odontoclast differentiation for root resorption. Besides, inflammatory cytokines produced by monocytes/macrophages such as Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α) also exhibited boneresorbing activity [21,22]. From early days, it has been recognized that orthodontic force may have an effect on apical dental pulp during orthodontic tooth movement. It was reported that circulatory disorders in pulp depend on the amount of orthodontic force [23]. Spector et al. demonstrated that orthodontic treatment may cause pulp necrosis, which was a non-infectious reaction similar to tooth trauma [24]. Spurrier et al. stated that vital teeth showed severe root resorption in contrast with teeth with endodontic treatment under orthodontic force [25]. However, the relationship between root resorption and pulp reaction during orthodontic tooth movement has not been proved yet. Although most reports about orthodontic root resorption focused on periodontal tissue, Kaku et al. suggested that root canal treatment is effective for managing progressive severe root resorption during tooth movement [26]. It was shown that IL-1β, TNF-α, RANKL and M-CSF were highly expressed when human pulp cells were subjected to excessive mechanical stimuli. Based on these findings, we hypothesized that the dental pulp tissue under orthodontic force may express inflammatory cytokines and osteoclast related factors. As a result, internal apical root resorption occurs because of derived odontoclasts. In this study, we examined the expression of inflammatory cytokines IL-1β and TNF-α, and osteoclast differentiation inducing factors RANKL, M-CSF during experimental tooth movement using immunohistochemical analysis. We also investigated whether the existence of pulp tissue influences the degree of root resorption under orthodontic force in rats.
Subjects and Methods
Animals
The animal experimental protocol in this study was approved by the Ethics Committee for Animal Experiments at Hiroshima University. A total of 12 male, 7-week-old, Wistar strain rats (Charles River Laboratories International, Inc., Tokyo, Japan) weighting 200 ± 10 g were used for this experiment. The rats were divided into three groups (four rats in each group). (1) Right first molar was subjected to pulpectomy and tooth movement (10 g pulp less group, the number of teeth=4) and left first molar was subjected to mesial movement by 10 g force application (10 g vital group, the number of teeth=4). (2) Right first molar was subjected pulpectomy and tooth movement (50 g pulp less group, the number of teeth=4) and left first molar was subjected to mesial movement by 50 g force application (50 g vital group, the number of teeth=4). (3) Right first molar was subjected pulpectomy without tooth movement (pulp less NT, the number of teeth=4) and left first molar was served as control (vital NT, the number of teeth=4).
The rats were kept in the animal center of Hiroshima University in separate cages, in a 12 hour light/dark environment at a constant temperature of 23°C, and provided with food and water ad libitum.
Pulpectomy and root canal treatment
Pulpectomy and root canal filling of the right maxillary first molar tooth were performed. By using a #1/2 round bur drilled to the depth of pulp, followed by instrumentation of the mesial, mid-buccal and distopalatal canals with #25 K-file endodontic instrument, pulp was extracted and the root canals were irrigated with sterilized saline solution 0.85%. The root canals were dried with absorbing paper points (Zipperer, Munich, Germany) and filled with gutta-percha points (Dentsply Sankin, Tokyo, Japan) and sealer (Shofu Inc., Kyoto, Japan). The condition of root canal filling was evaluated by dental radiographic imaging.
Application of orthodontic devices
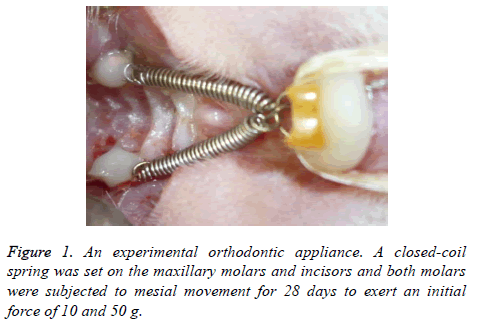
An experimental appliance with a closed-coil spring was bonded onto the upper first molars and incisors. A closed-coil spring (wire size: 0.009 × 0.030 inch, Rocky mountain morita Co., Tokyo, Japan) was ligated to the maxillary molar and incisors with a 0.008 inch stainless steel ligature wire (Figure 1, Tomy International Inc., Tokyo, Japan). Both molars were subjected to mesial movement for 28 days. The force magnitude was calibrated by a tension gauge (YDM Co., Tokyo, Japan) to exert an initial force of 10 and 50 g.
Tissue preparation
After 28 days, rats were sacrificed under general anaesthesia with sodium pentobarbital. Specimens were fixed in 4% paraformaldehyde, were decalcified in 14% EDTA (pH 7.4) for 28 days, and were embedded in paraffin. Pre-maxillary bones, including the upper molars, were cut into frontal sections of 5 μm in thickness in the sagittal direction.
The amount of root resorption and the number of TRAP positive cells
For evaluating the amount of internal root resorption at the apex area (measuring range: 700 × 1200 μm2), five sections from each sample were used. Digitized photomicrographs were captured using software on a computer (NIH Image, Bethesda, MD). The histological sections were stained with tartrateresistant acid phosphatase (TRAP) and counterstained with haematoxylin. Odontoclasts were identified as TRAP-positive and multi-nucleated cells. The odontoclasts that appeared at the root apex were counted on five sections at 35 μm intervals for each specimen. Because it is difficult to distinguish pulp tissue from Periodontal Ligament (PDL), the measuring area was defined as the part among internal wall of the root (measuring range: 700 × 1200 μm2). The amount of mesial movement of the first molar was measured using an orthodontic calliper (Dentech Co. Tokyo, Japan) in each group after 28 days of tooth movement.
Immunohistochemistry
Immunohistochemical staining was performed as follows: the sections were deparaffinised and endogenous peroxidase activity was quenched by incubation in 3% H2O2 in methanol for 30 minutes at room temperature. After washing in Phosphate Buffered Saline (PBS), the sections were incubated with polyclonal rabbit-anti-human IL-1β (abcam; working dilution, 1:50), polyclonal goat-anti-human TNF-α (R & D systems, Inc.; working dilution: 1:50), polyclonal rabbit-antihuman RANKL (abcam; working dilution, 1:100), and polyclonal goat-anti-human M-CSF (Santa Cruz Biotechnology; working dilution: 1:50) overnight at 4°C. IL-1β, TNF-α, RANKL and M-CSF were stained using Histofine Simple Stain MAX-Po kits (Nichirei, Tokyo, Japan) according to the manufacturer's protocol.
The sections were rinsed with PBS and final color reactions were performed using the substrate reagent 3, 3’- diaminobenzidine tetra-hydrochloride and aminoethylcarbazole and then counter-stained with haematoxylin. The number of positive cells that appeared at the root apex was determined to be the same as the number of TRAP positive cells (measuring range: 700 × 1200 μm2).
Statistical analysis
Data were expressed as mean ± standard error of the mean. We used Student’s t-test to evaluate statistical differences in the amount of root resorption and the number of odontoclasts. For quantification of IL-1β, TNF-α, RANKL and M-CSF positive cells, statistical significance was evaluated using analysis of variance followed by Fisher’s procedure. A confidence level of p<0.05 was defined as statistically significant.
Results
The amount of root resorption
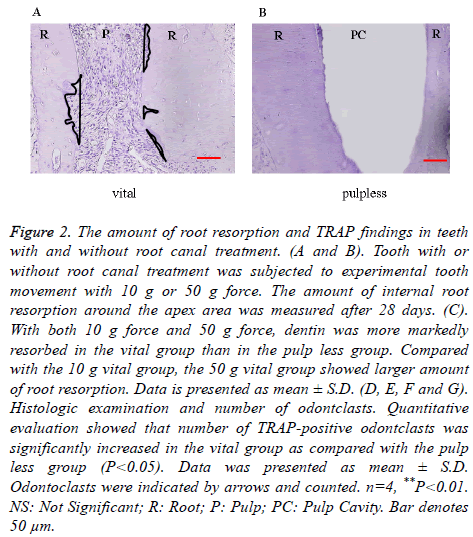
After experimental tooth movement for 28 days, internal root resorption at the apex area was observed both in vital and pulp less teeth. In both the 10 g force group and 50 g force group, dentin was more markedly resorbed in the vital group than in the pulp less group. The internal root resorption area in the 10 g vital group was around 4500 μm2, but was around 2000 μm2 in the 10 g pulp less group, showing a significant difference. The internal root resorption area in the 50 g vital group was around 10000 μm2, whereas it was around 2000 μm2 in the 50 g pulp less group. There was a significant difference between these two groups. The 50 g vital group showed larger amount of internal root resorption compared with the 10 g vital group. There was no significant difference in the amount of root resorption between the 50 g pulp less group and the 10 g pulp less group (p<0.05, Figures 2A-2C). The internal root resorption area was around 0 μm2 in the vital NT group and pulp less NT group. The amount of tooth movement was around 0.6 mm, with no significant difference between the vital and pulp less group, irrespective of force magnitude.
Figure 2. The amount of root resorption and TRAP findings in teeth with and without root canal treatment. (A and B). Tooth with or without root canal treatment was subjected to experimental tooth movement with 10 g or 50 g force. The amount of internal root resorption around the apex area was measured after 28 days. (C). With both 10 g force and 50 g force, dentin was more markedly resorbed in the vital group than in the pulp less group. Compared with the 10 g vital group, the 50 g vital group showed larger amount of root resorption. Data is presented as mean ± S.D. (D, E, F and G). Histologic examination and number of odontclasts. Quantitative evaluation showed that number of TRAP-positive odontclasts was significantly increased in the vital group as compared with the pulp less group (P<0.05). Data was presented as mean ± S.D. Odontoclasts were indicated by arrows and counted. n=4, **P<0.01. NS: Not Significant; R: Root; P: Pulp; PC: Pulp Cavity. Bar denotes 50 μm.
TRAP-positive cells on the root apex
In the 10 g and 50 g force groups, some resorption lacunae with TRAP-positive multinucleated odontoclasts appeared at the root apex (Figures 2D and 2E). Quantitative evaluation showed that the number of TRAP-positive odontoclasts was significantly larger in the vital group as compared with the pulp less group (p<0.05, Figures 2F and 2G). More odontoclasts were observed in the 50 g vital group compared to the 10 g vital group.
Immunohistochemical staining of IL-1β, TNF-α, RANKL and M-CSF in vital teeth
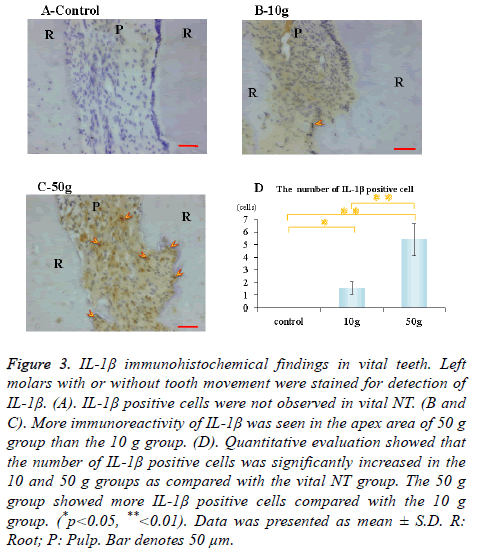
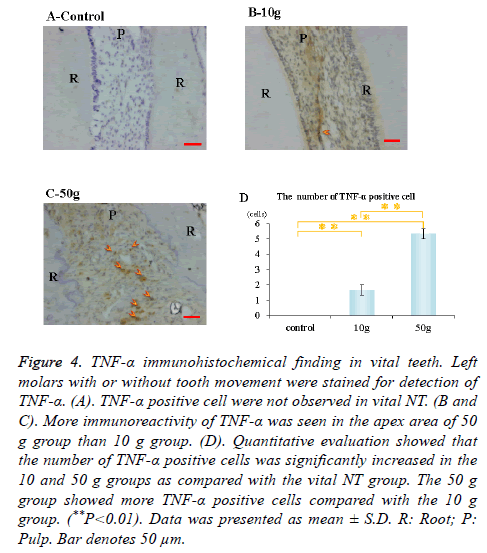
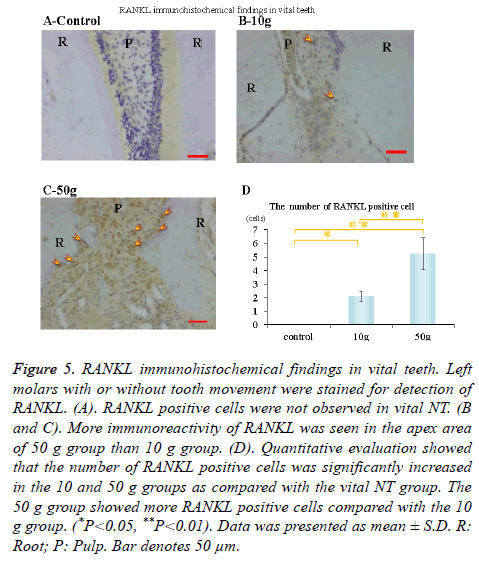
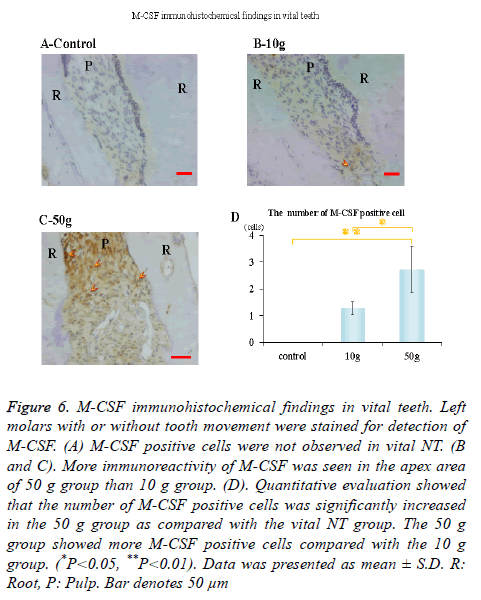
Immunohistochemical activity of IL-1β, TNF-α, RANKL and M-CSF was investigated 28 days after tooth movement in the vital group. In both 10 g group and 50 g group, immunereactivity of IL-1β (Figures 3B and 3C), TNF-α (Figures 4B and 4C), RANKL (Figures 5B and 5C) and M-CSF (Figures 6B and 6C) was localized in the root apex. Meanwhile, IL-1β, TNF-α, RANKL and M-CSF positive cells were not observed at any part of vital NT group (Figures 3A, 4A, 5A and 6A). Quantitative evaluation showed that the number of IL-1β, TNF-α and RANKL positive cells increased as orthodontic force become strong. The number of IL-1β, TNF-α and RANKL positive cells were significantly larger in the 10 and 50 g groups as compared with the vital NT group. There were significantly more M-CSF positive cells in the 50 g group as compared with the vital NT group (*p<0.05, **<0.01, Figures 3D, 4D, 5D and 6D).
Figure 3. IL-1β immunohistochemical findings in vital teeth. Left molars with or without tooth movement were stained for detection of IL-1β. (A). IL-1β positive cells were not observed in vital NT. (B and C). More immunoreactivity of IL-1β was seen in the apex area of 50 g group than the 10 g group. (D). Quantitative evaluation showed that the number of IL-1β positive cells was significantly increased in the 10 and 50 g groups as compared with the vital NT group. The 50 g group showed more IL-1β positive cells compared with the 10 g group. (*p<0.05, **<0.01). Data was presented as mean ± S.D. R: Root; P: Pulp. Bar denotes 50 μm.
Figure 4. TNF-α immunohistochemical finding in vital teeth. Left molars with or without tooth movement were stained for detection of TNF-α. (A). TNF-α positive cell were not observed in vital NT. (B and C). More immunoreactivity of TNF-α was seen in the apex area of 50 g group than 10 g group. (D). Quantitative evaluation showed that the number of TNF-α positive cells was significantly increased in the 10 and 50 g groups as compared with the vital NT group. The 50 g group showed more TNF-α positive cells compared with the 10 g group. (**P<0.01). Data was presented as mean ± S.D. R: Root; P: Pulp. Bar denotes 50 μm.
Figure 5. RANKL immunohistochemical findings in vital teeth. Left molars with or without tooth movement were stained for detection of RANKL. (A). RANKL positive cells were not observed in vital NT. (B and C). More immunoreactivity of RANKL was seen in the apex area of 50 g group than 10 g group. (D). Quantitative evaluation showed that the number of RANKL positive cells was significantly increased in the 10 and 50 g groups as compared with the vital NT group. The 50 g group showed more RANKL positive cells compared with the 10 g group. (*P<0.05, **P<0.01). Data was presented as mean ± S.D. R: Root; P: Pulp. Bar denotes 50 μm.
Figure 6. M-CSF immunohistochemical findings in vital teeth. Left molars with or without tooth movement were stained for detection of M-CSF. (A) M-CSF positive cells were not observed in vital NT. (B and C). More immunoreactivity of M-CSF was seen in the apex area of 50 g group than 10 g group. (D). Quantitative evaluation showed that the number of M-CSF positive cells was significantly increased in the 50 g group as compared with the vital NT group. The 50 g group showed more M-CSF positive cells compared with the 10 g group. (*P<0.05, **P<0.01). Data was presented as mean ± S.D. R: Root, P: Pulp. Bar denotes 50 μm
Discussion
Many previous reports have investigated the relationship between force magnitude and amount of tooth movement. It is generally considered that light orthodontic force produces more tooth movement [27]. When subjected to orthodontic force, tooth movement occurs in three distinct phases. The initial phase is viscoelastic deformation of the periodontal ligament and alveolar bone. This is followed by a lag phase in which stagnation of the movement of the teeth occurs due to the advent of glass-like degeneration organization. The final phase is tooth movement due to the loss of the modified organization and bone remodeling [28]. It is suggested that compression of the PDL, existence of hyalinised tissue, as well as direct or undermining bone resorption on the pressure side may regulate the rate of tooth displacement [29]. On the other hand, orthodontic force is indicated as a cause of root resorption. It is thought that external root resorption during orthodontic treatment occurs by direct contact of root and alveolar bone. Many researchers reported that root resorption is worsened with an increase of force magnitude [30,31]. Gonzales et al. showed that with a light force application of 10 g, the movement of significantly larger teeth showed notable less root absorption over a period of 28 days compared to heavy force application in rats [27]. The optimal force for movement of the rat upper molars has been reported to be less than 10 g [29]. Therefore, we examined the molar mesial movement by 10 and 50 g force application, and observed that 50 g force induced larger number of odontoclasts and more internal root resorption at the root apex area. On the other hand, some authors investigated the relation between pulp tissue and root resorption. After orthodontic treatment, it was reported that root resorption in pulpectomized tooth was less than that of vital teeth [25,32], although the mechanism was not clear. Graber [33] stated that severe orthodontic force can impair the apical vessels, and Salzmann [34] also reported that orthodontic force may cause pulpal disturbance as a result of tipping of the teeth with stretched apical blood vessels. McDonald et al. stated that continuous application of tipping force reduced pulpal blood vessels [35]. It was also suggested that continuous heavy orthodontic forces lead to pulp necrosis by the rupture of blood vessels at the apical part of the root [36]. Thus, it is easy to speculate that inflammatory reactions occur in the apical pulp during orthodontic treatment. Odontoclasts induce resorption of the cementum and dentin through an acidization/degradation-associated pathway and the mechanisms of root resorption are similar to those of bone resorption by osteoclasts. Kodama indicated that the number of osteoclasts in osteopetrotic (op/op) mice can be increased by injections of recombinant human M-CSF, which cured osteopetrosis [37]. Therefore, it was indicated that M-CSF has an important role in osteoclast differentiation. RANKL is also crucial not only for osteoclast differentiation but also for osteoclast activation [38]. It was confirmed that M-CSF supports osteoclast differentiation in cooperation with RANKL [19]. Moreover, some cytokines can promote inflammation by enhancing osteoclast differentiation. A previous study showed that IL-1 stimulates osteoclast differentiation and boneresorbing activity [39]. It was reported that the fusion of monocytes into osteoclasts is regulated by TNF-α, although it inhibits osteoblast differentiation from progenitor cells [40]. Kaku et al. showed that after cyclic tensile force application in human pulp cells for 48 hours, gene expression and protein concentration of M-CSF, RANKL, IL-1β and TNF-α peaked with loading at 10 kPa tensile force [26]. The role of the Stretch-Activated channel (S-A) channel was also evaluated by gadolinium (Gd3+) treatment and it was observed that Gd3+ reduced the expression of these cytokine mRNAs and protein concentrations. Moreover, they showed that the amount of inflammatory root resorption was significantly larger in control teeth than pulpectomized teeth in rats followed by mesial movement for 6 months. These results suggested that tensile forces in the pulp cells enhance the expression of various cytokines via the S-A channel, which may lead to inflammatory root resorption during tooth movement and root canal treatment is effective for managing progressive severe inflammatory root resorption during tooth movement. Apical root resorption during orthodontic treatment is an unfavourable phenomenon and it is crucial to clear out the etiology. In the present study, we demonstrated the new theory that dental pulp tissue plays an important role in the process of root resorption during orthodontic tooth movement. Rustem et al. demonstrated that few inflammatory cells in human pulp tissue were detected 10 and 40 days after extrusion [41]. However, the authors did not focus on pulp tissue in the root apex. This may explain the difference in the results of our study. In this study, immunoreactivity of IL-1β, TNF-α, RANKL and MCSF was localized at the root apex both in 10 and 50 g groups, although positive cells of these factors were not observed in the non-tooth movement group. The numbers of IL-1β, TNF-α, RANKL and M-CSF positive cells were significantly larger in the 50 g group as compared with the control group (non-tooth movement group). Furthermore, the number of TRAP-positive odontoclasts was significantly larger in the vital group as compared with the pulp less group and the amount of internal root resorption at the apex area was larger in the vital group than in the pulp less group after experimental tooth movement. In the present study, we did not set a non-tooth movement for pulp less group. Mutoh et al. observed root apex tissue after root canal filling [42]. On day 7, moderate infiltration of inflammatory cells was observed in dense fibrous tissues with numerous blood vessels and amorphous materials were recognizable in the periodontal ligament. However, by day 14, inflammatory cells disappeared, and the periapical tissue showed normal periodontal tissue. According to these findings, it can be suspected that there is no difference between pulp less groups with or without tooth movement. Our findings in this study strongly suggest that stretched and injured pulp tissues in the root apex express IL-1β, TNF-α, RANKL and M-CSF during orthodontic treatment. As a result, internal apical root resorption may occur because of derived odontoclasts during clinical orthodontic tooth movement. Furthermore, our results indicated that light orthodontic forces should be applied to prevent severe orthodontically induced inflammatory root resorption.
Conclusion
In this study, we showed that the number of TRAP-positive odontoclasts was significantly larger in the vital group as compared with the pulp less group. The amount of internal root resorption was significantly larger in the vital group than in the pulp less group. Furthermore, during orthodontic tooth movement, pulp tissue in the root apex express IL-1β, TNF-α, RANKL and M-CSF. Therefore, during orthodontic tooth movement, injured pulp cells express these bones resorbing cytokines; as a result, internal apical root resorption may occur because of derived odontoclasts.
Funding
This study was funded by JSPS KAKENHI Grant Number 25862019 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Massler M, Malone AJ. Root resorption in human permanent teeth: a roentgenographic study. Am J Orthod Dentofacial Orthop 1954; 40: 619-633.
- Weltman B, Vig KW, Fields HW, Shanker S, Kaizer EE. Root resorption associated with orthodontic tooth movement: A systematic review. Am J Orthod Dentofacial Orthop 2010; 137: 462-476.
- Marques LS, Ramos-Jorge ML, Rey AC, Armond MC, Oliveira Ruellas AC. Severe root resorption in orthodontic patients treated with the edgewise method: Prevalence and predictive factors. Am J Orthod Dentofacial Orthop 2010; 137: 384-388.
- Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod 1983; 5: 173-183.
- Beck BW, Harris EF. Apical root resorption in orthodontically treated subjects: analysis of edgewise and light wire mechanics. Am J Orthod Dentofacial Orthop 1994; 105: 350-361.
- Alexander SA. Levels of root resorption associated with continuous arch and sectional arch mechanics. Am J Orthod Dentofacial Orthop 1996; 110: 321-324.
- Levander E, Malmgren O. Evaluation of the risk of root resorption during orthodontic treatment: a study of upper incisors. Eur J Orthod 1988; 10: 30-38.
- Levander E, Malmgren O, Stenback K. Apical root resorption during orthodontic treatment of patients with multiple aplasia: a study of maxillary incisors. Eur J Orthod 1998; 20: 427-434.
- Brin I, Tulloch JF, Koroluk L, Philips C. External apical root resorption in Class II malocclusion: a retrospective review of 1- versus 2-phase treatment. Am J Orthod Dentofacial Orthop 2003; 124: 151-156.
- Sasamoto T, Motokawa M, Matsuda Y, Kaku M, Kawata T, Ozaki N, Terao A, Tanne K. Clinical survey on the risk factors of root resorption in orthodontic treatment. J Hiroshima Univ Dent Soc 2011; 43: 113-123.
- Reitan K. Initial tissue behaviour during apical root resorption. Angle Orthod 1974; 44: 68-82.
- Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofacial Orthop 1993; 103: 62-66.
- Matsuda Y, Motokawa M, Kaku M, Kawata T, Yamamoto R, Tsuka N, Inubushi T, Sasamoto T, Ozaki N, Koseki H, Kawazoe A, Abedini S, Tanne K. Clinical survey of the association between root resorption incident to orthodontic treatment and host factors. Orhod Waves 2011; 70: 21-31.
- Hall AM. Upper incisor root resorption during stage II of the Begg technique: two case reports. Br J Orthod 1978; 5: 47-50.
- Sasaki T. Differentiation and functions of osteoclasts and odontoclasts in mineralized tissue resorption. Microsc Res Tech 2003; 61: 483-495.
- Udagawa N. Mechanisms involved in bone resorption. Biogerontol 2002; 3: 79-83.
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337-342.
- Tsukii K, Shima N, Mochizuki N, Yamaguchi K, Kinosaki M, Yano K, Shibata O, Udagawa N, Yasuda H, Suda T, Higashio K. Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1a, 25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun 1998; 246: 337-341.
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin / osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998; 95: 3597-3602.
- Ross FP, Teitelbaum SL. Alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 2005; 208: 88-105.
- Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res 1991; 26: 230-242.
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 2003; 74: 391-401.
- Seltzer S, Bender IB. The dental pulp. Philadelphia PA USA JB Lippincott (3rd edn.) 1990.
- Spector JK, Rothenhaus B, Herman RI. Pulpal necrosis following orthodontic therapy. Report of two cases. N Y State Dent J 1974; 40: 30-32.
- Spurrier SW, Hall SH, Joondeph DR, Shapiro PA, Reidel RA. A comparison of apical root resorption during orthodontic treatment in endodontically treated and vital teeth. Am J Orthod Dentofacial Orthop 1990; 97: 130-134.
- Kaku M, Sumi H, Shikata H, Kojima S, Motokawa M. Effects of pulpectomy on the amount of root resorption during orthodontic tooth movement. J Endod 2014; 40: 372-378.
- Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod 2008; 78: 502-509.
- King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop 1991; 99: 456-465.
- Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod 2002; 29: 129-136.
- Reitan K. Evaluation of orthodontic forces as related to histologic and mechanical factors. SSO Schweiz Monatsschr Zahnheilkd 1970; 80: 579-596.
- Chan E, Darendeliler MA. Physical properties of root cementum. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop 2005; 127, 186-195.
- Remington DN, Joondeph DR, Artun J, Riedel RA, Chapko MK. Long-term evaluation of root resorption occurring during orthodontic treatment. Am J Orthod Dentofacial Orthop 1989; 96: 43-46.
- Graber TM. Orthodontics: principles and practice. Philadelphia PA USA W B Saunders Co (2nd edn.) 1967.
- Salzmann JA. Practice of Orthodontics. Philadelphia PA USA JB Lippincott 1966.
- McDonald F, Pitt Ford TR. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod 1994; 16: 1-9.
- Miura F. Granada Publishing Limited. Effect of orthodontic force on blood circulation in periodontal membrane. Trans Int Orthodont Congr 1973; 1973: 35-41.
- Kodama H, Yamasaki A, Nose M, Niida S, Ohgame Y. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med 1991; 173: 269-272.
- Burgess TL, Qian Y, Kaufman S, Ring BD, Van G. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 1999; 145: 527-538.
- Jimi E, Nakamura I, Duong LT, Ikebe T, Takahashi N. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res 1999; 247: 84-93.
- Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene 2003; 321: 1-15.
- Rustem KS, Hakan K, Berna T, Arzu S, Charles F. Response of human pulpal tissue to orthodontic extrusive applications. J Endod 2001; 27: 508-511.
- Mutoh N, Satoh T, Watabe H, Tani-Ishii N. Evaluation of the biocompatibility of resin-based root canal sealers in rat periapical tissue. Dent Mater J 2013; 32: 413-419.