ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Health Science and Bio Convergence Technology: Edition-I
Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated thoracic aorta of ovariectomized rats
The present study was undertaken to investigate the effect of CAPE on isolated thoracic aorta of menopausal model rats. Fifty ovariectomized wistar-albino rats randomly divided into eight groups (n=6-7); CAPE 10 μM endothelium (+), CAPE 10 μM endothelium (-), CAPE 100 μM endothelium (+), CAPE 100 μM endothelium (-), CAPE 300 μM endothelium (+), CAPE 300 μM endothelium (-), ethanol and endothelium (+), ethanol and endothelium (-). After hanging into a heated organ bath, aorta rings were first stretched by 1 gram and then stretched to 4 gm in 1 gm increments in 10 minutes episodes. When the tension records were stable, contraction response using 0.1 ml NE 10-4 M and relaxation response using 0.1 ml Ach 10-4 M were obtained. In the experiment phase, 0.1 ml NE 10-4 M was delivered first into each of the baths. Once contraction was reached to plateau level, 10 μM, 100 μM, 300 μM CAPE were delivered into each bath respectively. In vehicle control group, responses were observed by giving thirty per cent of ethanol into the baths. There was a significant dose dependent increase in relaxation of the aorta rings with healthy and damaged endothelium. The response of CAPE 10 endothel (-) group was significantly different from the response of CAPE 300 endothel (+) group. Other groups did not display any significant difference. In the light of these results, we believe that this study can lead the way for the future work on the treatment of the cardiovascular problems of the menopause period.
Keywords
CAPE, Isolated organ baths, Aorta, Relaxation response, Menopause.
Introduction
Menopause is the permanent cessation of menstruation resulting from loss of ovarian follicular activity and is diagnosed retrospectively following 12 months of amenorrhea in association with elevated gonadotropins and oestrogen deficiency [1]. Animal models of menopause uses ovariectomized rats as these animals lack female hormone oestrogen.
Oestrogen causes vasodilatation on the vascular system. It also prevents atherosclerosis, and increases angiogenesis and collateral branches [2]. The effect of oestrogen on vasodilatation in normal vessels follows synthesis of NO which plays a major role on endothelial functions [3]. After releasing from the endothelial cells, NO diffuses into vascular smooth muscle cells, and causes vascular relaxation via cGMP activation. In vessels deprived of endothelial cells however vasodilatation effect of oestrogen occurs via Ca+2 activated K+ channels [4].
Caffeic acid phenethyl ester (CAPE) also has vasodilator effect on the vascular system. Unlike oestrogen, the detailed mechanism of CAPE induced vasodilatation is not yet certain. It is claimed that CAPE does the vasodilatation effect via NO in vessels with intact endothelium using the Ca+2 channels. Its effect in the endothelium damaged vessels however is said to be via β-adrenergic receptors [5-7]. However, it is not known however how CAPE’s relaxation effect occurs during menopause.
To date, studies have shown the vaso-relaxant effect of CAPE. However no previous study has investigated the effect of menopause on this issue. In this study we hypothesize that CAPE’s effect continues to exist during and after menopause. To explore this hypothesis, this study examined CAPE’s effect in the thoracic aorta in ovariectomized rats with and without intact endothelium.
Materials and Methods
Chemicals
Caffeic acid phenethyl ester (CAPE) and ethanol were purchased from the Sigma-Aldrich Corporation (St.Louis, MO). CAPE was dissolved in ethanol.
Animals
Wistar-Albino female rats (250-300 g) were kept in a lightcontrolled room [12:12h (light: dark) photoperiod) and temperature-(22 ± 0.5ºC) maintained at a constant relative humidity of 65-70% and fed a standard diet and water ad libitum. All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at Adnan Menderes University. Bilateral ovariectomies were performed under intra peritoneally administered ketamine and xylazine HCl anaesthesia using a dorsal approach in all animals; the rats were kept for 1 month to recover and adapt to the absence of endogenous oestrogen. The 1-month duration for ovariectomized animals was previously used in the same experimental model successfully, and it has been reported that this protocol produced postmenopausal changes in ovariectomized animals [8-10].
Methods
Fifty ovariectomized Wistar-Albino rats randomly divided into eight groups (n=6-7); CAPE 10 μM endothelium (+) group (I), CAPE 10 μM endothelium (-) group (II), CAPE 100 μM endothelium (+) group (III), CAPE 100 μM endothelium (-) group (IV), CAPE 300 μM endothelium (+) group (V), CAPE 300 μM endothelium (-) group (VI), ethanol endothelium (+) group (VII), ethanol endothelium (-) group (VIII ) [n.b., negative ‘-‘ indicates groups with damaged endothelium. Rats were anaesthetized using ketamine (50 mg/kg)+xylazine HCI (5 mg/kg). Chest cavity was surgically exposed from the bottom left side of the sternum.
The heart and the lungs were moved to left side to expose the thoracic aorta. The thoracic aorta and aortic arch were carefully extirpated and placed in dish that had Krebs-Henseleit solution. After the dissection of fatty and other tissues around the aorta, it was sliced into 3-5 mm rings. The rings were quickly hung into a heated organ bath that contained carbonated Krebs- Henseleit solution. Aorta rings were first stretched by 1 gram and then stretched to 4 gr in 1gr increments in 10 minutes episodes. When the tension records on a computer monitor were stable, contraction response using NE 10-4 M and relaxation response using 0.1 ml ACh (10-4) were obtained. Finally the preparation was washed twice using Krebs-Henseleit solution and the experiment was initiated 45 minutes later. During the experiment 0.1 ml NE 10-4 M was delivered into each of the baths and contraction responses of the aorta were observed. Once stable plateau contraction level was reached, 0.1 ml of CAPE in 10 μM, 100 μM, 300 μM doses were delivered into each of the three groups of the baths (3 × 4). Thirty percent of ethanol that is used to dissolve CAPE was prepared as a vehicle group and delivered in 0.1 ml volumes into the baths.
Statistical analysis
Values of the percentage relaxation were tested for normal distribution using the Kolmogorov-Smirnov test. Since each group’s distribution was normal, we have used one way ANOVA test for comparing the groups. However, since variances were not homogenous, we have also used Tamhene test for multiple comparisons. For statistical significance we have accepted p value less than 0.05. IBM SPSS version 14.0 was used for all statistical analysis.
Results
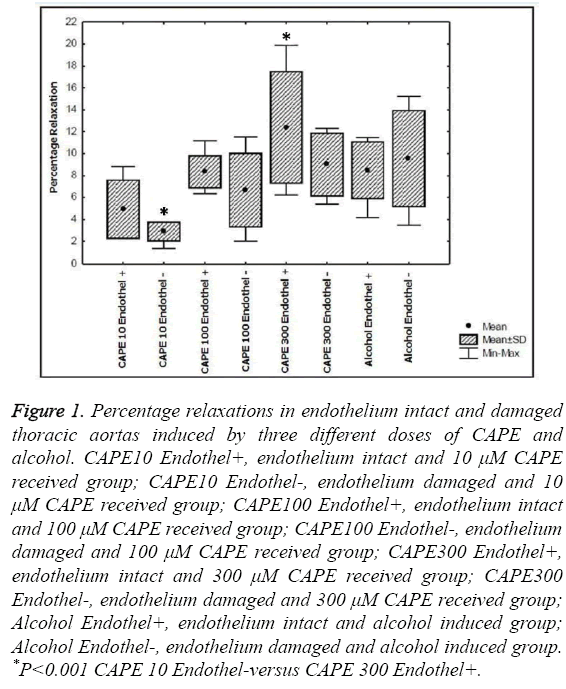
Table 1 illustrates the percentage relaxations of endothelium intact (endothelium +) and endothelium damaged (endothelium -) thoracic aorta induced by CAPE (in three different doses: 10, 100, 300 μM) and by alcohol used to dissolve CAPE.
| Percentage relaxation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group Name | n | Mean | Standard deviation | Standard error | 95% confidence interval | Group | ||
| Min | Max | Min | Max | |||||
| CAPE10 Endothel+ | 7 | 4.93 | 2.70 | 1.02 | 2.43 | 7.42 | 2.27 | 8.80 |
| *CAPE 10 Endothel - | 6 | 2.89 | 0.88 | 0.36 | 1.96 | 3.81 | 1.33 | 3.65 |
| CAPE 100 Endothel+ | 7 | 8.34 | 1.49 | 0.57 | 6.95 | 9.72 | 6.33 | 11.18 |
| CAPE 100 Endothel- | 6 | 6.66 | 3.40 | 1.39 | 3.09 | 10.23 | 2.03 | 11.52 |
| * CAPE 300 Endothel+ | 6 | 12.38 | 5.12 | 2.09 | 7.01 | 17.76 | 6.22 | 19.89 |
| CAPE 300 Endothel- | 6 | 9.00 | 2.90 | 1.18 | 5.96 | 12.05 | 5.39 | 12.28 |
| Alcoholendothel+ | 6 | 8.48 | 2.63 | 1.08 | 5.72 | 11.24 | 4.15 | 11.43 |
| Alcoholendothel- | 6 | 9.54 | 4.43 | 1.81 | 4.89 | 14.20 | 3.48 | 15.21 |
| Total | 50 | 7.73 | 4.02 | 0.57 | 6.59 | 8.87 | 1.33 | 19.89 |
Table 1: Statistical parameters for percentage relaxations in endothelium intact and damaged thoracic aortas induced by three different doses of CAPE and alcohol.
There was a statistically significant difference between groups regarding percentage relaxation (p<0.001). Specifically, there was a statistically significant difference in the percentage relaxation between endothelium damaged group that received 10 μM CAPE (2.89 ± 0.88) and endothelium intact group that were given 300 μM CAPE (12.38 ± 5.12) (p<0.001). No further significant difference was found.
Figure 1 illustrates percentage relaxations in endothelium intact and damaged thoracic aortas induced by three different doses of CAPE and alcohol.
Figure 1: Percentage relaxations in endothelium intact and damaged thoracic aortas induced by three different doses of CAPE and alcohol. CAPE10 Endothel+, endothelium intact and 10 μM CAPE received group; CAPE10 Endothel-, endothelium damaged and 10 μM CAPE received group; CAPE100 Endothel+, endothelium intact and 100 μM CAPE received group; CAPE100 Endothel-, endothelium damaged and 100 μM CAPE received group; CAPE300 Endothel+, endothelium intact and 300 μM CAPE received group; CAPE300 Endothel-, endothelium damaged and 300 μM CAPE received group; Alcohol Endothel+, endothelium intact and alcohol induced group; Alcohol Endothel-, endothelium damaged and alcohol induced group. *P<0.001 CAPE 10 Endothel-versus CAPE 300 Endothel+.
As can be clearly seen, the only significant difference was between the lower dose+endothelium damaged group and the high dose+endothelium intact group.
Therefore, both the dose of CAPE and the health of endothelium seem to be factors for the relaxation of the aorta. Figure 1 also shows that the usage of the dissolve substance (ethanol) did not significantly alter the relaxation procedure of the thoracic aorta.
Discussion
Until now, the development of the positive effects of oestrogen on vascular cells and the speed of these effects has been associated with oestrogen receptors. Estradiol generates fast aortic vasodilatation via mechanisms related to oestrogen receptors and nitric oxide (NO). Oestrogen induced fast release of endothelial NO plays a key role in the establishment of the vascular homeostasis [11].
In our study, we have induced experimental menopause on rats by surgically removing their ovaries (oophorectomy). We have then extirpated their thoracic aorta rings and hung in organ baths. After testing three different doses of CAPE we obtained the maximal relaxation using 300 μM CAPE on the aorta rings with intact endothelium illustrated in Table 1 and Figure 1.
Cicala et al. investigated the effects of CAPE on male rat aortic rings that contained either healthy or damaged endothelium and generated contractions using phenylephrine and KCl. They found a concentration dependent inhibitory effect of CAPE on phenylephrine induced contraction of aortic rings with healthy endothelium. However, the KCI induced contractions could only be inhibited by high doses of CAPE [5]. Phenylephrine induces contraction via Ca+2 increase either via specific channels or release from the intracellular pool. High level of KCI concentration increases Ca+2 entry via voltage sensitive Ca+2 channels and induces contraction [12]. The contraction inhibitory effect of high concentration CAPE may be via either Ca+2 receptors or via voltage sensitive channels. In this study, they applied CAPE (1-100 μM) on endothelium either after incubating in L-NAME (N-nitro-L-arginine methyl ester) or similar to the method used in our work, after mechanically damaging and lifting the endothelium. They observed that CAPE did not generate relaxation in low concentrations levels but it increased its relaxation property as the concentration level has been increased [5]. This is similar to our observations in the group with damaged endothelium as we have noted minimal relaxation at the lowest dose (10 μM) and the level of relaxation increased with increase in CAPE concentration (100 μM, 300 μM).
In the study of the CAPE’s effect on the endothelium intact aorta rings, Cicala et al. have put forward that its relaxation effect may occur as a result of a dual mechanism involving, the first; induction of NO release by CAPE when it is in low concentrations, the second; inhibition of Ca+2 entry to the cell and Ca+2 release from intracellular sources when it is in high concentrations [5].
In another study using CAPE on segments of pig coronary artery, it was observed that the relaxation effect of CAPE was significantly reduced in segments with damaged endothelium. This finding provides additional evidence that the relaxation effect of CAPE on the pig coronary artery occurs via the endothelium NO-cGMP pathway. After been released from the endothelial cells, NO stimulates guanylate cyclase and increase cGMP level in vascular smooth muscle cell. cGMP then activates cGMP dependent protein kinase, which subsequently phosphorylates troponin-I and decreases contraction of vascular smooth muscle. In this study, it has been also shown that the CAPE induced relaxation is reduced after blocking β- adrenergic receptors, suggesting that CAPE’s effect can also be via β-adrenergic receptors and cAMP activation, which inhibits Ca+2 channels in vascular smooth muscle membranes [6].
Our findings on the CAPE’s effect on aorta rings with intact endothelium suggest that while CAPE’s relaxation effect in low concentration may involve NO release, it is related to its inhibitory effect on the Ca+2 entry into cell through the cell membrane at higher concentration. These findings, similar to the findings of Cicala et al. support a dual mechanism that underlay the relaxation effect of CAPE as shown in Table 1 and Figure 1 [5]. In the light of our results, we are now thinking of exploiting CAPE’s stimulating influence on NO release as an alternative treatment and prevention of cardiovascular diseases that may occur during menopause. We believe that this study can lead the way for the future work on the treatment of the cardiovascular problems of the menopause period.
References
- Teede HJ, Vincent A. Hormone therapy- where are we now. Aust Fam Physician 2011; 40: 280-285.
- Mendelson ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Eng J Med 1999; 340: 1801-1811.
- Mendelson ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol 2000; 74: 337-343.
- Mendelson ME. Protective effects of estrogen on the cardiovascular system. Am J Card 2002; 89: 12-17.
- Collins P, Rosano GM, Jiang C, Lindsay D. Cardiovascular protection by estrogen-a calcium antagonism effects. Lancet 1993; 341: 1264-1265.
- Cicala C, Morello S, Iorio C, Capasso R. Vascular effects of caffeic acid phenethyl ester (CAPE) on isolated rat thoracic aorta. Life Sci 2003; 73: 73-80.
- Long Y, Han M, Chen J, Tian XZ. The vasorelaxant effect of caffeic acid phenethyl ester on porcine coronary artery ring segments. Vascul Pharmacol 2009; 51: 78-83.
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function andinhibition. Biochem J 2001; 357: 593-615.
- Nemcsik J, Morschl E, Egresits J, Kordas K. Raloxifene lowers ischemia susceptibility byincreasing nitric oxide generation in the heart of ovariectomized rats in vivo. Eur J Pharmacol 2004; 495:179-184.
- Konyalioglu S, Durmaz G, Yalcin A. The potential antioxidant effect of raloxifene treatment: a study on heart, liver and brain cortex of ovariectomized female rats. Cell Biochem Funct 2007; 25: 259–-66.
- Ek RO, Serter M, Ergin K, Yıldız Y. The effects of caffeic acid phenethyl ester (CAPE) on TNBS-induced colitis in ovariectomized rats. Dig Dsi Sci 2008; 53: 1609-1617.
- Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen reseptors. Steroids 2008; 73: 864-869.
- Godfraind M, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev 1986; 38: 321-416