ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
VDR stimulation improves outcome of isoprenaline-induced myocardial infarction in rats via down-regulation of cardiac inos gene expression
Atef M. Abood1,3*, Mohamed Farouk Elshal2,4
1Physiology Department, Faculty of Medicine
2Biochemistry department, Faculty of sciences, King Abdulaziz University, Saudi Arabia
3Physiology Department, Faculty of Medicine, Ain Shams University
4Molecular Biology department, Genetic Engineering and Biotechnology Institute, Sadat City University, Egypt.
- *Corresponding Author:
- Atef M. Abood
Faculty of Medicine
King Abdulaziz University
Jeddah, Saudi Arabia
Accepted : May 19 2015
Coronary heart disease has been reported to become a leading cause of death by the end of this decade. Isoprenaline-induced myocardial infarction is a well-known model to study myocardial infarction which is characterized by inflammation and oxidative stress. Meanwhile, vitamin D has been reported to have anti-inflammatory and antioxidant effects. The present study has been designed to investigate the effect of vitamin D receptor stimulation by a vitamin D receptor agonist, paricalcitol, on the outcome of isoprenaline (ISO) induced myocardial infarction in rats. Forty rats were divided into four equal groups (n= 10) for a 4-week experimental period. The first group (C) consisted of normal rats, the second group: normal rats injected with paricalcitol (C+P) at a dose of 200ng/kg 3 times a week for four weeks, these rats were used as a positive control. The third group consisted of rats injected with isoprenaline at a dose of 100mg/kg for the last 2 successive days of the study period, and the fourth group, isoprenaline-injected rats pretreated with paricalcitol. The same injection protocols for PC and isoprenaline were followed in the 4th group. The results showed that the ISO group exhibited significant ECG changes, cardiac enzymes and a histopathological picture of myocardial infarction. Pretreatment with PC before ISO injection resulted in minimal changes in the heart evidenced by near normal ECG, cardiac enzymes and histopathology. The serum tumour necrosis factor-α, Interlukin-6, as well as cardiac malondialdehyde and nitrites were significantly higher in ISO-treated rats compared to normal rats. Similarly, the cardiac content of inducible nitric oxide synthase messenger RNA was significantly elevated in ISO-injected animals. However, these parameters in rats subjected to ISO injection pretreated with PC were not significantly different from their counterpart controls. In conclusion, VDR stimulation with paricalcitol appears to have a protective action against isoprenaline-induced myocardial infarction via exerting anti-inflammatory and antioxidant effects, together with the down-regulation of cardiac inducible nitric oxide synthase gene expression.
Keywords
Vitamin D, myocardial infarction, iNOS, TNF-α
Introduction
Cardiovascular diseases are the principal threat to health in countries in Africa and the Middle East, as elsewhere. According to the WHO, cardiovascular diseases represent 42% of the causes of death in Egypt and 35% in Saudi Arabia. According to the WHO reports, coronary heart disease (CHD) is going to be the major cause of death in the world by 2020 [1,2].
Isoprenaline (ISO), a β adrenergic agonist, has been reported to produce a reliable, reproducible, and wellcharacterized model of myocardial infarction when given in large doses. It has been shown to exhibit many metabolic and morphological aberrations in the heart tissue of experimental animals similar to those seen in humans with myocardial infarction [3]. Injected isoprenaline undergoes autooxidation, and generates highly cytotoxic free radicals known to stimulate the peroxidation of membrane phospholipids causing severe damage [3,4]. Inflammation is a key process involved in mediating myocardial tissue damage. Neutrophils infiltrate the infracted area where they can promote myocardial cell damage through the release of proteolytic enzymes and the production of reactive oxygen species (ROS) [5], which aggravate the inflammatory response and apoptosis of cardiomyocytes [6].
Vitamin D, a well-known hormone which regulates calcium and phosphorus metabolism, has emerged as a promising cardio protective agent. Compelling data in the last decade about the cardio protective effects of vitamin D have been reported [7,8]. The pivotal role of vitamin D was more evidenced when reports about its deficiency were linked to cardiovascular deleterious effects. Low levels of calcidiol, as well as calcitriol, were associated with endothelial dysfunction [9]. Moreover, vitamin D deficiency was associated with major adverse cardiovascular outcomes immediately following an acute myocardial infarction (MI) [10].
Furthermore, it was reported that PC (a known VDR agonist) treatment resulted in both prevention of progression of pre-existing cardiac hypertrophy and development of heart failure [11]. More recently, Yao and colleagues [12] identified a role for VDR stimulation in the amelioration of myocardial ischemic reperfusion injury. Two effects of vitamin D have attracted attention in this area of research, its anti-inflammatory and anti-oxidant actions [13,14].
Despite contradictory reports in the literature regarding the association between vitamin D levels and inflammatory markers in patients with acute coronary syndrome [15], vitamin D was found to down-regulate proinflammatory cytokines such as interleukin-1 (IL-1), interleukin- 6 (IL-6) and tumor necrosis factor alpha (TNF- α). It also upregulate the anti-inflammatory cytokine interleukin- 10 (IL-10) [13].Moreover, this antiinflammatory effect was demonstrated in tissues other than the cardiovascular system [16]. Although there is accumulating data about the cardiovascular effect of vitamin D, a direct role of vitamin D in acute myocardial infarction in a powered controlled study is not available in the literature. Considering the anti-inflammatory and antioxidant effects of vitamin D, we hypothesize that vitamin D may play a role in protecting the myocardium against inflammatory and oxidative changes during acute infarction. Hence, the present study was designed to investigate the effect of pre-supplementation with vitamin D receptor agonist (paricalcitol) on the outcome of ISOinduced myocardial infarction in rats, and to outline a molecular mechanism for this effect if found.
Materials and Methods
Animals
Forty male Wistar albino rats weighing 150–200g were used. The animals were purchased from the Egyptian Organization for Biological Products and Vaccines (Cairo, Egypt). Rats were housed in plastic cages in the animal house of the Department of Physiology, Faculty of Medicine, Ain Shams University. They were allowed free access to water and standard pellet diet, and kept under standard conditions with 12/12 h light/dark cycles. The study was carried out according to the guidelines of the Ethics Committee, Faculty of Medicine, Ain Shams University.
Experimental work
Rats were randomly allocated into four groups (n =10). Group I, normal rats (C), Group II, PC treated rats and served as positive control group (C+PC). Group III consisted of isoprenaline-injected rats (ISO), Group IV, isoprenaline- injected rats pretreated with paricalcitol (ISO+PC). Groups I and III received the vehicle of PC for 4 weeks and served as normal and control groups, respectively. Groups II and IV were given paricalcitol (PC) 200ng/Kg 3 times a week for 4 weeks. Isoprenaline hydrochloride and Vitamin D agonist paricalcitol (PC) were purchased from Sigma–Aldrich (MO, USA)
Paricalcitol was prepared with 95% propylene glycol/5% ethyl alcohol solution and injected intraperitoneally (i.p.). Administration of 200ng of PC three times per week was chosen after extensively reviewing previous reports where doses of up to 250 ng i.p. three times per week were safely administered without significant changes in blood pressure or serum calcium or phosphorus. Moreover, PC (19-nor-1,25-(OH)2 D2), is ≈10 times less potent than the endogenous form of active vitamin D (calcitriol) regarding the gastrointestinal absorption of calcium and phosphorus [17]. For induction of MI, rats in groups III and IV received isoprenaline (100mg/kg; s.c.) on the last 2 days of the treatment period. Isoprenaline hydrochloride and Vitamin D agonist paricalcitol (PC) were purchased from Sigma–Aldrich (MO, USA). Myocardial infarction was induced in rats by subcutaneous injection of 100mg/ kg isoprenaline hydrochloride dissolved in saline once daily for two successive days. The selected route and dose were chosen from published literature (Kumaran and Prince 2010) [18].
Twenty-four hours after the last treatment, overnightfasted rats were weighed, anaesthetized with i.p. injection of pentobarbitone (40mg/ kg) and underwent ECG recording. Anesthetized rats were placed in the supine position on a board and ECG was recorded continuously with standard artifact-free lead II (right forelimb to left hind limb). Needle electrodes were inserted subcutaneously into the paw pads of each rat, and connected to Biocare ECG 101 (Shenzhen Biocare Electronics Co., Ltd., China). The ECG was measured to determine duration of QRS complex, R-R interval, R voltage, Q-Tc intervals and S-T segment elevations.
A mid-line abdominal incision was then made and wholeblood specimen from the abdominal aorta was collected and centrifuged at 5000 rpm for 15 minutes. Separated serum was stored at -20°C for later biochemical analysis of alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), cardiac troponin I (cTn-I) and creatine kinase (CK-MB) activity , in addition to the inflammatory markers TNF-α, and IL-6. Hearts were excised after blood collection and washed with isotonic saline. Hearts were divided into 2 halves one half was frozen and stored in liquid nitrogen (N2) for biochemical assays, the other half was preserved in 10% formalin for histopathologic examination.
Biochemical assays
The activity of ALT, AST, LDH, and CK-MB were determined using Stanbio CK-MB diagnostic kit (USA). Serum cTn-I levels were measured by enzyme linked immunoassay (ELISA) technique using a standard kit (Glory Science Co., Ltd, USA). Lipid peroxidation in cardiac tissue was evaluated by measuring malondialdehyde (MDA) in heart homogenate using a standard kit purchased from Biodiagnostic (Egypt).
Cardiac nitrite was determined as an index of nitric oxide (NO) content in heart homogenate using a commercial kit (Biodiagnostic, Egypt). Serum TNF-α and IL-6 were determined by ELISA technique using standard kits (Ray Biotech, Inc., USA).
RT-PCR for Identification of iNOS-mRNA in cardiac homogenates was carried out by measuring total RNA of cardiac tissues homogenate was extracted by using TRIzol Reagent (Invitrogen, Life Technologies, USA) according to the manufacturer's instructions. Reverse transcription was carried out with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). PCR was performed with Taq DNA polymerase. The following primers were used for detecting iNOS: 5′-GGGATGGCTTGCCCCTGG-3′ and 5′- CGGAGGCAGCACATCAAAG-3′. Primers 5′-GGTGA AGG TCG GA G T C AACG-3′ and 5′- CAAAGTTGTCATGGATGACC-3′ were used for measuring GAPDH.
Histopathological studies
Small pieces from the left ventricle were immediately fixed in 10% formol saline. Paraffin blocks were prepared and 5μm sections were cut and stained with hematoxylin and eosin (H & E). Other specimens from the left ventricle were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer solution (pH 7.4) and post-fixed in 1% osmium tetroxide. Ultrathin sections were cut and mounted on copper grids, stained with uranyl acetate and lead acetate, and examined under Zeiss, EM10 transmission electron microscope (Carl Zeiss, Oberkochen, Germany) operated at 80KV at the National Research Center, Cairo, Egypt.
Statistical analysis
Data were expressed as mean ±SEM. Comparisons between means of different groups were carried out using one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test. The level of significance was taken as p <0.05. SPSS statistical software package, version 20 (SPSS Inc., Chicago, IL) was used to carry out all statistical tests.
Results
Histological Results
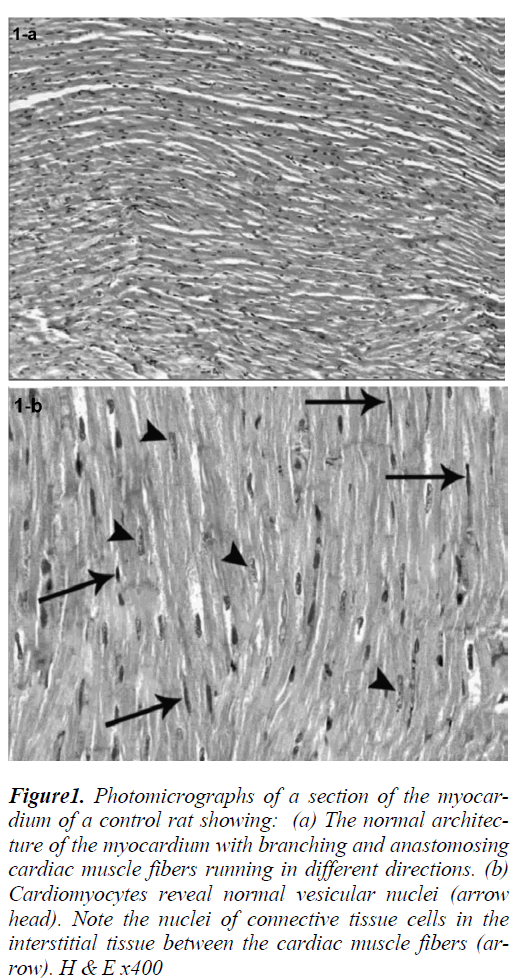
Sections of the left ventricular wall of normal animals showed the normal architecture of the myocardium with branching and anastomosing cardiac muscle fibers running in different directions (Fig. 1a) with cardiomyocytes showing normal vesicular nuclei. Nuclei of connective tissue cells could be noticed in the interstitial tissue between the cardiac muscle fibers (Fig. 1b).
Figure 1: Photomicrographs of a section of the myocardium of a control rat showing: (a) The normal architecture of the myocardium with branching and anastomosing cardiac muscle fibers running in different directions. (b) Cardiomyocytes reveal normal vesicular nuclei (arrow head). Note the nuclei of connective tissue cells in the interstitial tissue between the cardiac muscle fibers (arrow). H & E x400
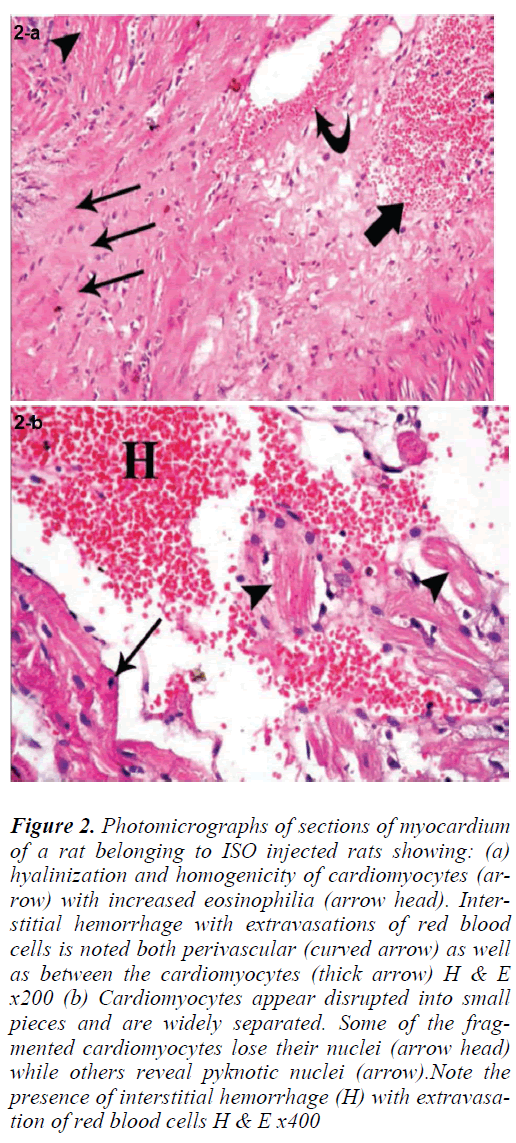
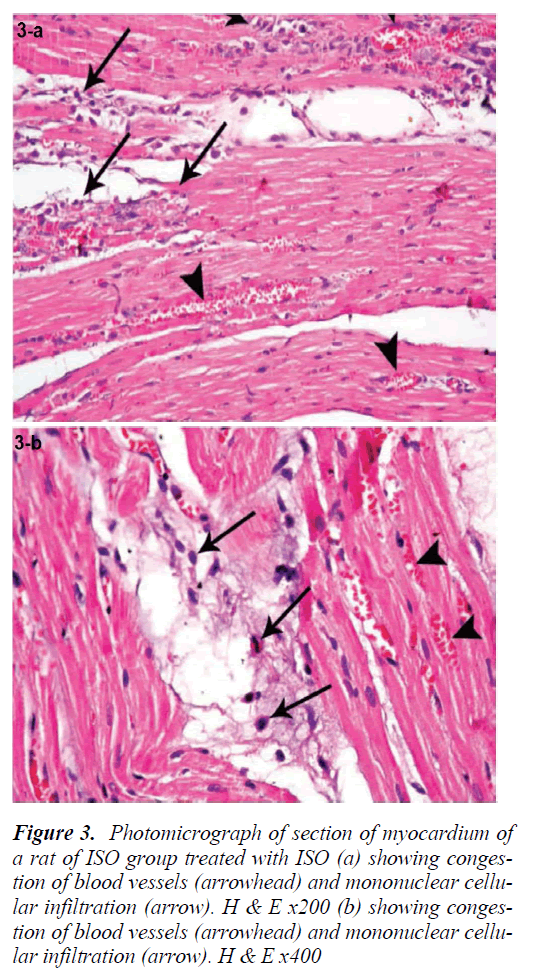
Sections of the left ventricular wall from rats injected with ISO alone showed foci of hyalinization and homogeneity of cardiomyocytes with increased eosinophilia. Fragmentation of cardiac muscle fibers was noted in other foci (Fig. 2a). Some of the fragmented cardiomyocytes lost their nuclei, while others revealed pyknotic nuclei. Cardiac muscle fibers appeared widely separated by empty spaces. Extravasation of red blood cells was seen both perivascular (Fig. 2b) as well as between the cardiomyocytes There was also infiltration with mononuclear cells, and some of the blood vessels appeared congested (Figs. 3a, 4b).
Figure 2: Photomicrograph of section of myocardium of a rat of ISO group treated with ISO (a) showing congestion of blood vessels (arrowhead) and mononuclear cellular infiltration (arrow). H & E x200 (b) showing congestion of blood vessels (arrowhead) and mononuclear cellular infiltration (arrow). H & E x400
Figure 3: Photomicrographs of sections of myocardium of a rat belonging to ISO injected rats showing: (a) hyalinization and homogenicity of cardiomyocytes (arrow) with increased eosinophilia (arrow head). Interstitial hemorrhage with extravasations of red blood cells is noted both perivascular (curved arrow) as well as between the cardiomyocytes (thick arrow) H & E x200 (b) Cardiomyocytes appear disrupted into small pieces and are widely separated. Some of the fragmented cardiomyocytes lose their nuclei (arrow head) while others reveal pyknotic nuclei (arrow).Note the presence of interstitial hemorrhage (H) with extravasation of red blood cells H & E x400
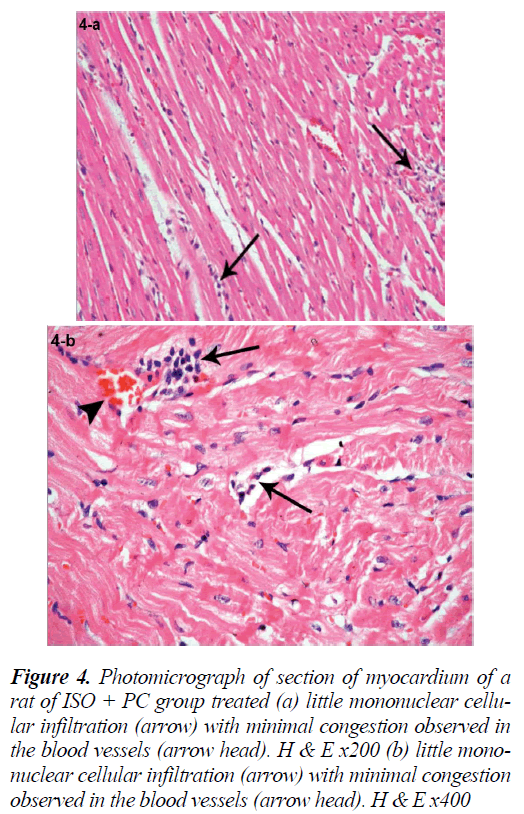
Figure 4: Photomicrograph of section of myocardium of a rat of ISO + PC group treated (a) little mononuclear cellular infiltration (arrow) with minimal congestion observed in the blood vessels (arrow head). H & E x200 (b) little mononuclear cellular infiltration (arrow) with minimal congestion observed in the blood vessels (arrow head). H & E x400
Sections of the left ventricular wall from rats pretreated with PC revealed a histological picture similar to that of normal rats. The foci of myocardial fragmentation, interstitial edema and mononuclear cellular infiltration were hardly detected in different sections. Congestion and hemorrhage were also less frequently observed (Fig. 4a, b).
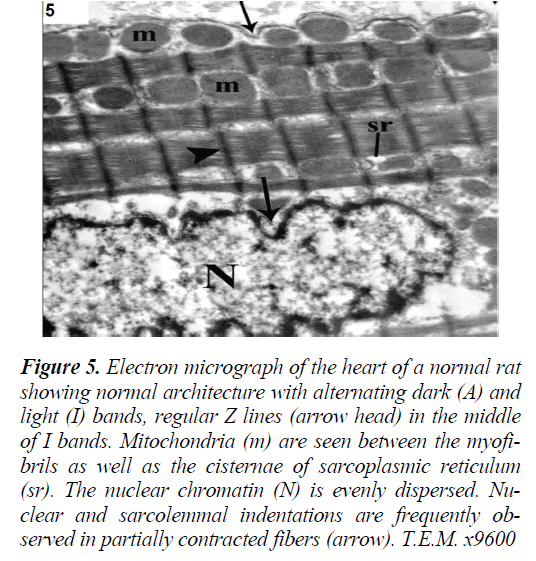
Electron microscopy of the left ventricular wall of control rats revealed normal architecture with alternating dark (A) and light (I) bands and regular Z lines in the middle of I bands. The nuclear chromatin was evenly dispersed. Nuclear and sarcolemmal indentations were frequently observed in partially contracted fibers (Fig. 5). On the other hand, Group II (ISO-treated group) demonstrated focal disruption and irregular arrangement of myofibrils with discontinuity of A bands and Z lines.
Figure 5: Electron micrograph of the heart of a normal rat showing normal architecture with alternating dark (A) and light (I) bands, regular Z lines (arrow head) in the middle of I bands. Mitochondria (m) are seen between the myofibrils as well as the cisternae of sarcoplasmic reticulum (sr). The nuclear chromatin (N) is evenly dispersed. Nuclear and sarcolemmal indentations are frequently observed in partially contracted fibers (arrow). T.E.M. x9600
Moreover, mitochondria appeared variable in size and shape and were mostly swollen. Some clear intermyofibrillar spaces were frequently noted. The cisternae of sarcoplasmic reticulum exhibited areas of dilatation (Fig. 6 a and b).
Figure 6: Electron micrograph of the heart of a rat of ISO group showing (a) disruption of myocardial fibers with variability in size and shape of mitochondria (m). The cisternae of sarcoplasmic reticulum exhibit areas of dilatation. (sr) T.E.M. x9600 (b)interruption and fragmentation of myofibrils. Focal disappearance of clear distinction between the A and I bands, lost I bands with obscured Z lines are noticed. Mitochondria (m) appear variable in size and shape but mostly swollen. The cisternae of sarcoplasmic reticulum are dilated. (sr). T.E.M. x9600
Specimens from ISO-injected rats pretreated with PC showed regular arrangement of the myofibrils with regular Z lines and normal appearance of mitochondria and sarcoplasmic reticulum (Fig. 7).
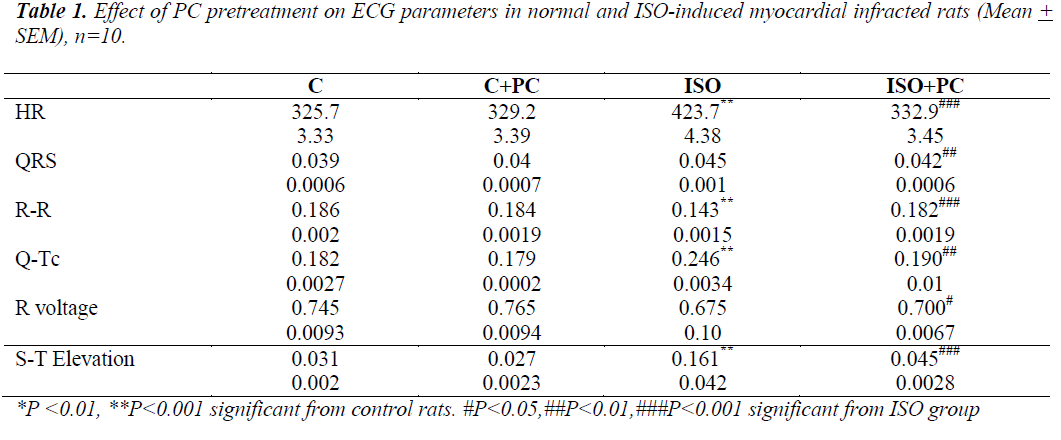
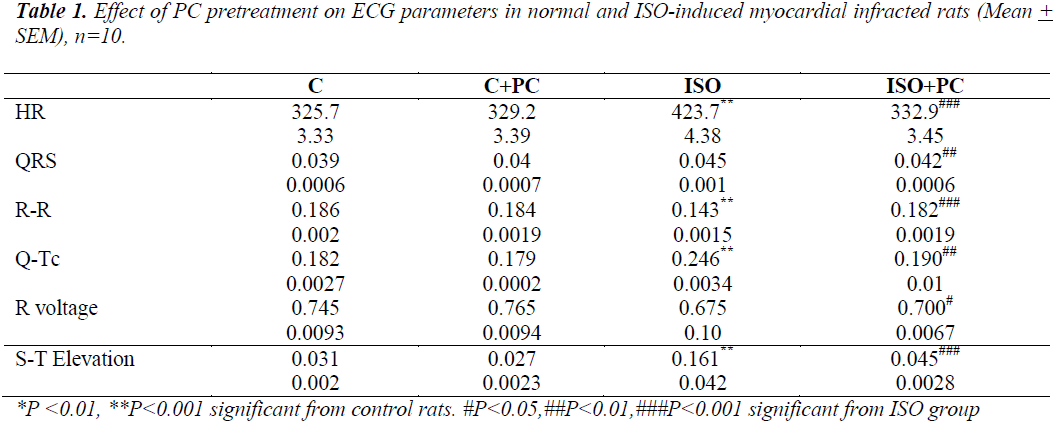
ECG results (table 1)
Compared to normal rats, ISO-injected rats showed a significant increase in HR and Q-Tc duration. There was also tion of the S-T segment. On the other hand, ISO-injected rats pretreated with PC showed no significant ECG changes from their counterpart controls.
a significant decrease in R-R intervals, as well as eleva
Biochemical results
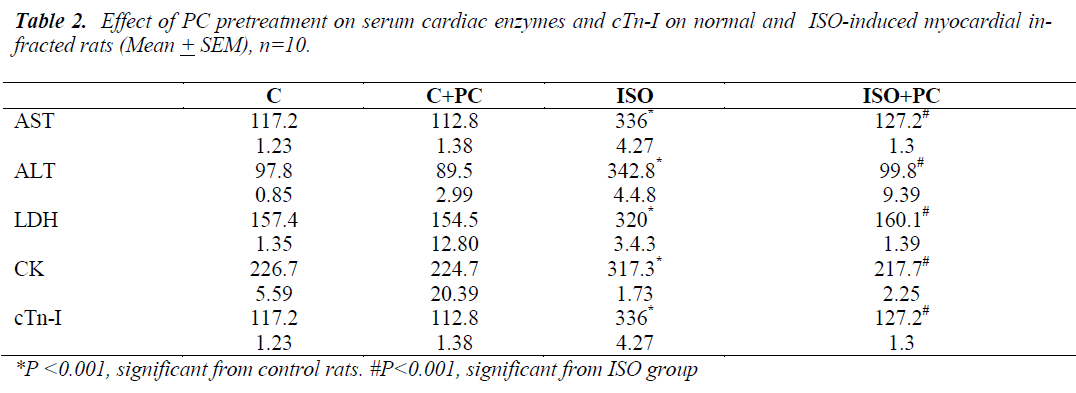
Table 2 depicts the levels of cardiac marker enzymes (ALT, AST, LDH, CK-MB and cTn-I) in the serum of normal and experimental groups of rats. Subcutaneous administration of isoprenaline caused a significant (p˂0.001) elevation in the levels of cardiac enzymes in the plasma compared with normal control rats. Pretreatment with PC 200ng/kg body weight for a period of 4 weeks significantly reduced the cardiac enzymes to near normal values.
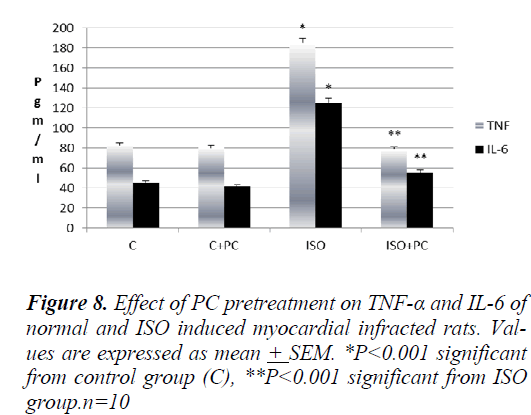
As regard pro-inflammatory cytokines, TNF-α and IL-6 increased significantly in ISO-treated rats (p˂0.001) compared to normal rats. However, these cytokines showed significant reduction in ISO-injected rats pretreated with PC (p˂0.001) compared to the ISO group. Moreover, these markers in the ISO + PC group were insignificantly different from their values in the control group (Fig. 8).
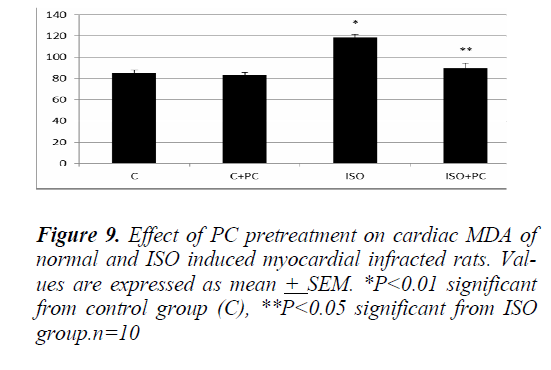
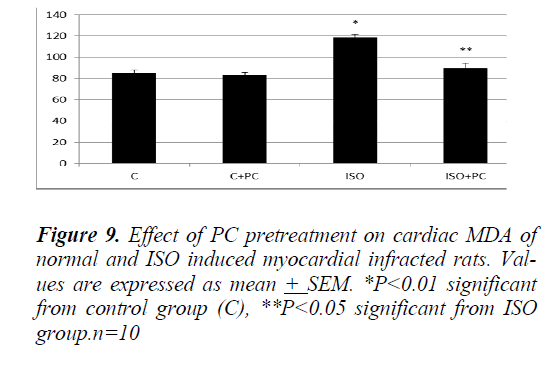
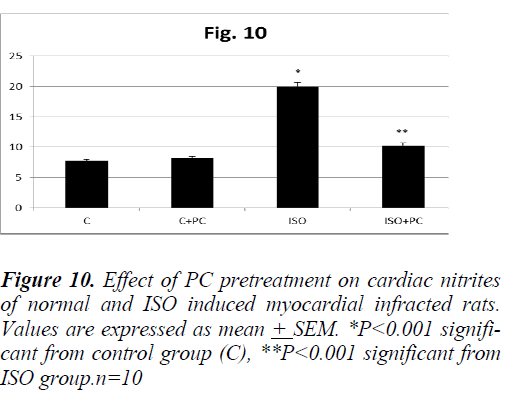
Similarly, the changes in MDA and nitrites showed a significant increase (p˂0.01, p˂0.001, respectively) in ISOinjected rats as compared to normal rats. On the other hand, pretreatment of the ISO group with PC resulted in a significant decline in the previous parameters when compared to animals treated with ISO alone (p˂0.05 and ˂0.001, respectively). Furthermore, cardiac MDA and nitrites parameters were not significantly different between ISO group pretreated with PC and their counterpart controls (Fig. 9 and 10).
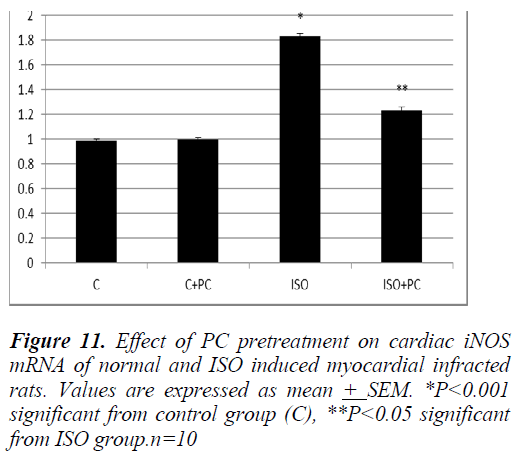
VDR stimulation improves outcome of isoprenaline-induced myocardial infarction….. iNOs-mRNA was significantly higher in rats injected with ISO alone compared to its level in normal animals (p˂0.001). On the contrary, pretreatment with PC of ISOinjected animals was associated with a significant reduction of cardiac iNOs-mRNA when compared to rats injected with ISO alone. Again, ISO+PC group of animals had cardiac iNOs-mRNa values insignificantly different from the control group (Fig. 11).
Discussion
In the present study, MI was induced in rats by subcutaneous administration of isoprenaline at a dose of 100mg/ Kg body weight for 2 successive days. Stimulation of VDR by a vitamin D receptor agonist for four weeks before induction of infarction resulted in amelioration of cardiac injury. Specifically, we have shown improvement in ECG parameters, cardiac enzymes and histopathological picture of ISO-injected rats pretreated with the vitamin D agonist, PC.
In accordance with Wang and colleagues [19] the diagnosis of a successful model in our study was dependent upon 3 main criteria; ECG changes, histopathological changes, and elevated serum level of cardiac enzymes. The ECG patterns recorded in the ISO-injected rats in our study conform to a previous report by Tawfik and colleagues [20]. Similarly, the histopathological changes seen in our study are in line with previous observations [4]. Furthermore, the cardiac enzymes, as a marker for acute myocardial infarction were all significantly elevated in our model. Since theses enzymes are released from necrotic cells to the extracellular fluid upon the incidence of infarction, their high levels give another evidence for necrosis and infarction [19].
In the present work, evidence of cardio-protection against MI by VDR stimulation was based on three main measures; improvement of ECG criteria and histopathological findings, in addition to return of cardiac enzyme parameters to near normal.
To our knowledge, cardio-protection by VDR stimulation in rats subjected to ISO-induced myocardial infarction was not reported before. Nevertheless, a potential therapeutic effect has been attributed to vitamin D in other cardiovascular disorders. Calcitriol treatment in spontaneous hypertensive heart failure rats resulted in lower heart weight, myocardial collagen levels and left ventricular diameter, which resulted in improved cardiac output [7]. Moreover, a recent study reported a cardio-protective effect by VDR stimulation in I/R injury in rat myocardium (Yao et al., 2015) [12].
To explain the protective effect of VDR stimulation in isoprenaline-induced MI we measured plasma TNF-α and IL-6, as markers for inflammation, together with cardiac MDA and nitrites content, as markers for the oxidative state. The elevation of cytokines as evident in our study was previously reported by Uryash et al [21]. Exogenous TNF-α in both normoxic and hypoxic conditions increased the expression of several pro-inflammatory and acute-phase molecules such as IL-6 in isolated adipocytes [22], suggesting a positive feedback mechanism for accumulation of pro-inflammatory cytokines. The significant positive correlation between TNF-α and IL-6 (r =0.7) in our study supports this concept. During myocardial ischemia, TNF-α is released from macrophages, monocytes and mast cells as it is expressed and secreted in both cardiomyocytes and fibroblasts [23]. Cell signaling of TNF-α through TNFR1 exacerbates remodeling, hypertrophy and apoptosis in heart failure [24].
The down regulation of inflammatory cytokines demonstrated by VDR stimulation in ISO-injected rats demonstrates that VDR stimulation exerts its protective effect, at least in part, by an anti-inflammatory action [25]. On the contrary, Witham et al [26] showed no reduction of inflammatory cytokines after 8 weeks of vitamin D3 supplementation. This contradiction is probably due to genetic polymorphism that may modulate the response to vitamin D3 supplementation [27]. Apart from genetic variations, VDR plays an intrinsic inhibitory role in inflammation by activation of a pro-inflammatory transcription factor NF-kB which increases in response to increased TNF-α [28].
It appears that the elevated cardiac MDA and nitrites after ISO injection in the present study contribute to the cardiac injury. MDA is a stable lipid peoxidation product that reflects over production of ROS in ISO-injected rats [29]. Isoprenaline is well known to generate free radicals and to stimulate lipid peroxidation, which may be a causative factor for irreversible damage to the myocardial membrane [3]. VDR stimulation in ISO-injected rats in our study was associated with a significant reduction in lipid peroxidation products resulting in amelioration of the oxidative stress. This conforms to the recent data published by Cavalcante and colleagues [25] regarding the antioxidant effect of vitamin D. Moreover, nitric oxide (widely recognized as a mediator of vasodilation with important anti-inflammatory properties) was elevated in cardiac tissues of rats subjected to infarction in our study, in accordance with previous information [30]. Elevation of NO could create a nitrosative stress and generate the powerful oxidant molecule peroxynitrite which functions as an important trigger of myocardial necrosis and apoptosis in various cardiac pathologies [31]. Accummulation of NO induces oxidative DNA damage, which in turn leads to the activation of the DNA repair enzyme and consumption of cellular NAD+, ultimately leading to ATP depletion and necrotic cell death [32].
In the present study, increased production of NO in ISOinjected animals was associated with a significant increase of iNOS gene expression in the rat heart. This conforms to previous reports [30,33]. The iNOS is one of the most important NO donors, and is associated with numerous important pathophysiological processes in MI and heart failure [34]. We have shown that VDR stimulation in ISO-injected animals significantly decreased cardiac NO and iNOS-mRNA. This Down-regulation of cardiac iNOS gene expression by VDR stimulation represents a novel finding, although such effect was reported in other tissues [35]. Cell apoptosis and nitrosamine formation have previously been attenuated by selective iNOS inhibitors and in iNOS-knockout mice [36].
It can be speculated that VDR stimulation in our study down-regulated iNOS gene expression which, consequently reduced the production of cardiac NO, resulting in a further drop in the production of nitrosamine radicals in the rat heart. The rate of isoprenaline-induced free radi cal generation is thus reduced, leading to improved outcome of myocardial injury.
Conclusion
In conclusion, the present study addresses, and demonstrates that VDR stimulation with vitamin D analogue, PC, could improve the outcome of isoprenaline-induced myocardial infarction in rats. This protective effect could be explained on the basis of strong anti-inflammatory and antioxidant effects. We also demonstrate for the first time the up regulation of cardiac iNOS gene expression by VDR stimulation in infracted rat myocardium.
References
- World Health Organization The World Health Report–Changing History. 2004; 120-124
- World Health Organization Data and statistics: mortality and health status. Available from: http://www.who.int/research/en/. 2015; Accessed January 12, 2015.
- Panda VS, Naik SR, Evaluation of cardioprotectiveactivity of Ginkgo biloba and Ocimum sanctum in rodents. Altern Med Rev. 2009; 14: 161-171.
- Kannan MM, Quine SD, Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism. 2013; 62: 52-61
- Jordan JE, Zhao ZQ,Vinten-Johansen J, The role of neutrophils in myocardial ischemia-reperfusion injury.Cardiovasc Res. 1999; 43: 860-878
- Chacko SM, Khan M, Kuppusamy ML, et al., Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2009; 296:H1263-H1273
- Mancuso P, Rahman A, Hershey SD, et al., 1,25- Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/ ) rats independent of changes in serum leptin. J CardiovascPharmacol.2008; 5: 559-564
- Brandenburg VM, Vervloet MG, Marx N, The role of vitamin D in cardiovascular disease: From present evidence to future perspectives. Atherosclerosis. 2012; 225: 253-263
- London GM, Guerin AP, Verbeke FH, et a.,l Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am SocNephrol. 2007; 18: 613-620
- Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ, Vitamin D and prognosis in acute myocardial infarction.Int J Cardiol (2013); 168: 2341-2346
- Bae S, Singh SS, Yu H, Lee JY, Cho BR, and Kang PM, Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol. 2013; 114: 979–987
- Yao T, Ying X, Zhao Y, et al., Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal. 2015; 22: 633-650
- Zittermann A, KoerferR, Protective and toxic effects of vitamin D on vascular calcification: clinical implications. Mol Aspects Med. 2008; 29: 423-432
- Shab-Bidar S, Neyestani TR, Djazayery A, The interactive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. Eur J Clin Nutr. 2014; 69: 216-222
- Eren E, Ellidag HY, Yýlmaz A, Aydýn Ö, Yýlmaz N, No association between vitamin D levels and inflammation markers in patients with acute coronary syndrome. Adv Med Sci. 2015; 60: 89-93
- Mao L, Ji F, Liu Y, Zhang W, Ma X, Calcitriol plays a protective role in diabetic nephropathy through anti-inflammatory effects. Int J ClinExp Med. 2014; 7: 5437-4544
- Fryer RM, Rakestraw PA, Nakane M, Dixon D, BanforPN, Koch KA, Wu-Wong JR, Reinhart GA, Differential inhibition of renin mRNA expression by paricalcitoland calcitriol in C57/BL6 mice. Nephron Physiol.2007; 106: 76-81
- Kumaran KS, Prince PS, Caffeic acid protects rat heartmitochondria against isoproterenol-induced oxidativedamage. Cell Stress Chaperon. 2010; 15: 791-806
- Wang SB, TianS, Yang F, Yang HG, Yang XY, Du GH, Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarc- tion in rats. Eur J Pharmacol. 2009; 615: 125-132
- Tawfik MK, Ghattas MH, Abo-Elmatty DM, Abdel- Aziz NA, Atorvastatin restores the balance between pro-inflammatory and anti-inflammatory mediators in rats with acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2010; 14: 499-506
- Uryash A, Bassuk J, Kurlansky P, Altamirano F, Lopez JR, Adams JA, Non Invasive Technology That Improves Cardiac Function after Experimental Myocardial Infarction: Whole Body Periodic Acceleration (pGz). PLoS One. 2015; Mar 25; 10(3): e0121069
- Bhattacharya I, Domínguez AP, Drägert K, HumarR, Haas E, Battegay E, Hypoxia potentiates tumor necrosis factorαinduced expression of inducible nitric oxide synthase and cyclooxygenase-2 in white and brown adipocytes. BiochemBiophys Res Commun.2015; 461: 287-292
- Schulz R, TNFalpha in myocardial ischemia/- reperfusion: damage vs. protection. Journal of molecular and cellular cardiology. 2008; 45: 712-714
- Schulz R, Heusch G, Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation. 2009; 119: 1355-1357
- Cavalcante IG, Silva AS, Costa MJ, Persuhn DC, IssaCI, de Luna Freire TL, Gonçalves MD, Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. ExpGerontol. 2015; 66:10-16
- Witham MD, Adams F, Kabir G, Kennedy G, Belch JJ, Khan F, Effect of shortterm vitamin D supplementation on markers of vascular health in South Asian women living in UK: A randomised controlled trial. Atherosclerosis. 2013; 230: 293-299
- Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore- Lorenti C, Sikaris K, et al., Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and β-cell function in multiethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One. 2014; 9, e109607
- Szeto, FL, Sun J, Kong J, Duan Y, Liao A, Madara JL, Li YC, Involvement of the vitamin D receptor in the regulation of NF-kappaB activity in fibroblasts. J Steroid BiochemMol Biol. 2007; 103: 563-566
- Panda VS, Naik SR, Cardioprotective activity of Ginkgo bilobaPhyto-somes in isoproterenol-induced myocardial necrosis in rats: a biochemi- cal and histoarchitecturalevaluation. xpToxicolPathol.2008; 60: 397-404.
- Pinto VD, Cutini GJ, Sartorio CL, Paigel AS, VassalloDV, Stefanon I, Enhanced beta adrenergic response in rat papillary muscle by inhibition of inducible nitric oxide synthase after myocardial infarction. ActaPhysiol (Oxf). 2007; 190: 111-117
- Li D, Qu Y, Tao L, Liu H, Hu A, Gao F, Sharifi-AzadS, Grunwald Z, Ma XL, Sun JZ Inhibition of iNOS protects the aging heart against beta-adrenergic receptor stimulation induced cardiac dysfunction and myocardial ischemic injury. J Surg Res.2006; 131: 64-72
- Pacher P, Szabó C, Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. CardiovascDrug Rev. 2007; 25: 235-260
- Wang J, Hao L, Wang Y, et al., Inhibition of poly (ADP-ribose) polymerase and inducible nitric oxide synthase protects against ischemic myocardial damage by reduction of apoptosis. Mol Med Rep. 2014; 11: 1768-1776
- Gilson WD, Epstein FH, Yang Z, et al., Borderzonecontractile dysfunction is transiently attenuated and left ventricular structural remodeling is markedly reduced following reperfused myocardial infarction in inducible nitric oxide synthase knockout mice. J Am CollCardiol. 2007; 50: 1799-807
- Dursun E, Gezen-Ak D, Yilmazer S A, new mechanism for amyloid-â induction of iNOS: vitami zn D-VDR pathway disruption. J Alzheimers Dis. 2013; 36: 459-74.
- Mukhopadhyay P, Rajesh M, Bátkai S, et al., Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009; 296: H1466-H1483