ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Vitamin K2 analog menaquinone-7 shows osteoblastic bone formation activity in vitro
1Department of Orthopaedics, Kunming General Hospital of PLA, Daguan road, Kunming, China
2Department of Orthopaedic Surgery, Third Military Medical University Affiliated Southwest Hospital, Gaotanyan, Chongqing, China
#These two authors contribute to this work equally.
- *Corresponding Author:
- Yongqing Xu
Department of Orthopaedics
Kunming General Hospital of PLA, China
Accepted date: September 08, 2016
An increasing number of studies have shown that vitamin K may play a critical role in metabolism of bone. However, the anabolic effect of MK-7 on promoting bone formation needs to be further investigated. The present study investigates the effect of MK-7 on osteoblastic bone formation in femoral-diaphyseal and -metaphyseal tissues of 4-week-old rats (n=18). The rat tissue was cultured in Dulbecco’s modified Eagle’s medium supplemented with MK-7 (10-6M). The activity of alkaline phosphatase DNA content, and calcium content in diaphyseal and metaphyseal tissues were used as osteogenesis markers. The study showed that treatment with MK-7 significantly increased alkaline phosphatase activity, DNA content, and calcium content in the cultured diaphyseal and metaphyseal tissue; however, the activity was inhibited in the presence of epoxomicin (10-6 M). Culture of human osteoblastic cells SAOS-2 for 24 h in a serum-free medium containing MK-7 (10-6M) resulted in increased alkaline phosphatase activity, osteocalcin content, and DNA content, and the reaction was completely inhibited in presence of by epoxomicin. The study results indicate that MK-7 has a stimulatory effect on bone tissue and osteoblastic SAOS-2 cells in vitro and further clinical studies are required to pave way for the use of MK-7 in bone formation.
Keywords
Vitamin K, Bone formation, Menaquinone-7, SASO-2, Epoxomicin.
Introduction
The decrease is bone mass due to aging appears to be an inevitable phenomenon. The loss can be prevented by pharmacological agents and nutritional factors. An increasing number of studies have shown that vitamin K may play a critical role in metabolism of bone. Vitamin K is a nutritional factor and is required for the γ-carboxylation of osteocalcin. Osteocalcin is a Ca2+-binding, noncollagenous protein found in bone and found to contain vitamin-K-dependent amino acid, γ- carboxyglutamic acid, synthesized by the osteoblasts tissue [1,2]. Noncarboxylated osteocalcin cannot bind to hydroxyapatite in mineralized tissues [2,3]. There is growing evidence to suggest that supplementation of vitamin K may serve as a therapeutic agent in osteoporosis.
Vitamin K can be divided into 2 types: vitamin K1 (phylloquinone) and vitamin K2 (menatetrenone, MK-4). Vitamin K1 is a single compound and has a 2-methyl-1, 4- naphthoquinone ring with a phytyl side chain at the third position. By contrast, vitamin K2 is a series of vitamers with multiisoprene units [1-4] at the third position of the naphthoquinone. Vitamin K1 has been approved for coagulation disorders and has been extensively studied to understand its role in bone metabolism. Vitamin K1 has been shown to retard bone loss and improve bone health [4,5]. However, few studies have explored the role of vitamin K2 on bone metabolism. Like vitamin K1, vitamin K2 (menatetrenone) has the ability to enhance mineralization as well as increase the concentration of osteocalcin in cultured human osteoblasts [6].
Additionally, menatetrenone has been showed to inhibit ovariectomy-induced bone loss in rats and bone resorption, which may be because of its side chain [7,8]. Recently, it has been reported that menaquinone with 7 isoprene units (menaquinone-7, MK-7) directly stimulates calcification in the femoral-metaphyseal tissues of normal rats in vitro [9,10]. Studies have reported that MK-7 had similar action as MK-4 on bone calcification [10]. However, the role of MK-7 in promoting bone metabolism is not fully understood. Fermented soybeans have a high concentration of MK-7 [10]. Previous studies have showed that dietary intake of MK-7 has an anabolic effect on osteoblastic cells and bone tissues in vitro and prevents bone loss induced by ovariectomy in rats [11,12]. The current evidence indicate that MK-7 may possess property to prevent osteoporosis [13,14]. However, the anabolic effect of MK-7 on promoting bone formation needs to be further investigated. With this background this study was initiated to evaluate the stimulatory effect of MK-7 in osteoblastic cells.
Materials and Methods
Chemicals
For cell culture standard high glucose Dulbecco’s modified Eagle’s medium (DMEM) was procured from Lonza Ltd, (Basel, Switzerland). α-Modified Eagle’s Minimal Essential Medium (α-MEM) was procured from Sigma Aldrich (St. Louis, MO, USA). Other chemicals such as Fetal bovine serum (FBS), bovine serum albumin (BSA fraction V), were procured from Sigma Chemical Co. (St. Louis, MO, USA). Epoxomicin was purchased from Hangzhou BSAZ Biotech Co., Ltd., (Hangzhou, Zhejiang, China). High purity grade (99.5%) Menaquinone-7 (vitamin K2) was procured form Zhengzhou Debao Fine Chemical co., ltd (Zhengzhou, China.). Other chemicals and reagents unless stated were obtained from China Chem Source (HK) Co., Ltd. (Dalian, China).
Bone tissue culture
Eighteen male wistar rats (4 weeks old) were procured from Model Animal Research Center of Nanjing University (Nanjing, China). Animals were kept at a room temperature of 25°C and were fed commercial available rat feed, with free access to distilled water. Blood samples were obtained from heart puncture under anaesthesis. The femur bone was surgically removed and soaked in ice-cold 0.25 M sucrose solution. The soft tissue surrounding femur bone was removed and the diaphysis and metaphysis part were removed. After removing the bone marrow the metaphyseal and diaphyseal tissue were cut into small cubes. The bone tissue was cultured in high glucose containing DMEM supplemented with 0.25% BSA plus antibiotics (100 units penicillin and 100 μg streptomycin/ml of medium) [15]. Experimental medium contained either vehicle (1% ethanol) or menaquinone-7 (10-6 M). The media was free of essential metals and culture was maintained at standard culture condition with 5% CO2.
Determination of bone components
The alkaline phosphatase activity was measured as per protocol of Walter and Schutt [16] and the concentration of enzyme activity was expressed as μmol of p-nitrophenol liberated/min/mg protein. The protein concentration was determined as per the standard Lowry method [16]. The measurement of DNA content was performed as per the procedure described by Ceriotti [17]. Briefly, the bone tissue were first agitated with 4.0 ml ice-cold 0.1 NaOH solution for 24 h, this led to the homoginisation of the tissue. Both the diaphyseal and metaphyseal tissues were then dried for 24 h at 80°C and then digested in nitric acid at 100°C for 24 h. The calcium content (mg/g) was measured with the help of atomic absorption spectrophotometry [15].
Cell culture
Osteoblastic SAOS-2 cells were cultured at 37°C in a CO2 incubator in plastic dishes containing α-MEM supplemented with 10% FBS [18]. They were subcultured every 3 days using 0.2% trypsin plus 0.02% ethylenediaminetetraacetic acid (EDTA) in Ca2+/Mg2+-free phosphate-buffered saline (PBS). For experiments, about 1 × 104 cells per dish were cultured for 3 days to obtain confluent monolayers in 35-mm plastic dishes containing 2 ml of α-MEM with 10% FBS. After the cells were rinsed with PBS, the medium was exchanged for medium containing 0.1% BSA plus menaquinone-7 (10-6 M), and the cells were cultured further for appropriate periods of time. Cell viability was estimated by staining with trypan blue. After trypsinization using 0.2% trypsin plus 0.02% EDTA in Ca2+/Mg2+-free PBS, cell numbers were determined by an electronic particle counter.
Determination of Osteocalcin levels
The protein content (μg) per dish of the osteoblastic cells was measured by the Lowry method [16]. The secreted osteocalcin from the osteoblastic cells was assayed by double-antibody ELISA method using commercially available Kit (ThermoFischer, Waltham, Massachusetts, USA) as per the manufacturer’s protocol. The osteocalcin concentration was expressed as μg/mg of DNA.
Acid phosphatase 5, tartrate resistant (ACP5) staining
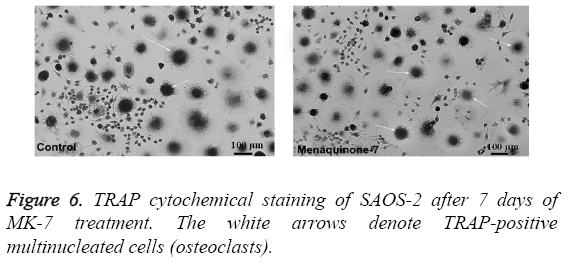
Human osteoblast-like cells (SASO-2) were cultured for 48 h and washed with PBS at the end of the time point and finally fixed with paraformaldehyde. The cells were then washed with miliQ water and stained with SAOS-2 cytochemical staining kit. After washing, the osteoblast cells with more than two nuclei were considered to be osteoclast-like cells. The number of osteoclast-like cells was counted under Leica DM4 P (Wetzlar, Germany), and the photomicrograph was obtained.
Statistical methods
Data is represented as mean ± SD from the triplicate values. Statistical analysis was performed using Origin Lab version 18.0 software (Origin Lab Inc., Guangzhou, China). Students’ non-paired t-test was measured to evaluate identical factors in relation to the sample and solvent control. P-value lesser than 0.001 were considered significant.
Results
Evaluation of outcome of MK-7 treatment on human osteoblast-like cells culture
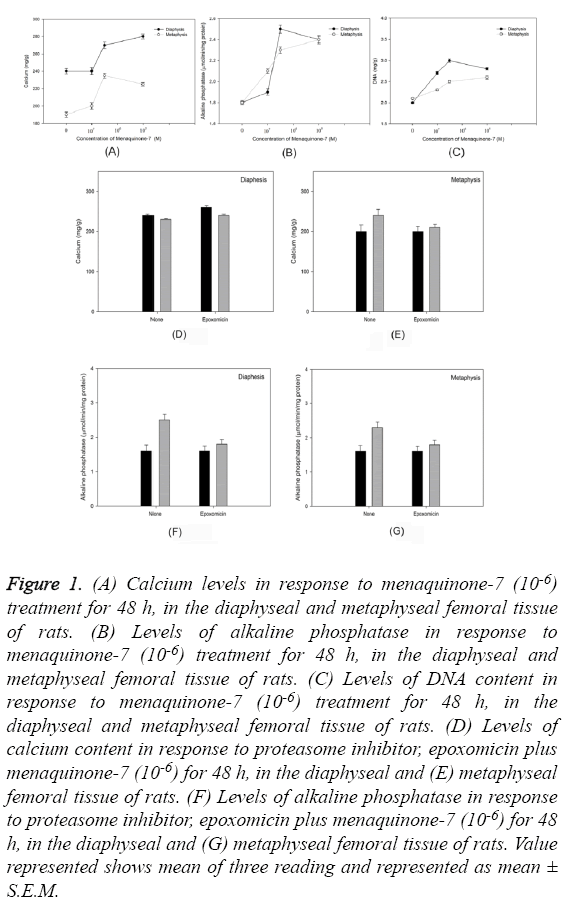
Culture of diaphyseal and metaphyseal tissue from femoral bone for 48 h in presence of menaquinone- 7 (10-6 M) revealed a statistically significant increase in the calcium content of the cultured cells (P=0.0002) (Figure 1A). Similar trend was observed in the alkaline phosphatase levels (P=0.00016) (Figure 1B) and DNA content (P=0.0006) (Figure 1C). Use of protein inhibitor eposomicin in SASO-2 cells cultured in presence of MK-7 (10-6 M) did not reveal similar trend of increase in calcium content and alkaline phosphatase levels in femoral tissue (both diaphyseal and -metaphyseal tissues) (Figures 1D-1G).
Figure 1: (A) Calcium levels in response to menaquinone-7 (10-6) treatment for 48 h, in the diaphyseal and metaphyseal femoral tissue of rats. (B) Levels of alkaline phosphatase in response to menaquinone-7 (10-6) treatment for 48 h, in the diaphyseal and metaphyseal femoral tissue of rats. (C) Levels of DNA content in response to menaquinone-7 (10-6) treatment for 48 h, in the diaphyseal and metaphyseal femoral tissue of rats. (D) Levels of calcium content in response to proteasome inhibitor, epoxomicin plus menaquinone-7 (10-6) for 48 h, in the diaphyseal and (E) metaphyseal femoral tissue of rats. (F) Levels of alkaline phosphatase in response to proteasome inhibitor, epoxomicin plus menaquinone-7 (10-6) for 48 h, in the diaphyseal and (G) metaphyseal femoral tissue of rats. Value represented shows mean of three reading and represented as mean ± S.E.M.
Effect of menaquinone-7 in osteoblastic cells in vitro
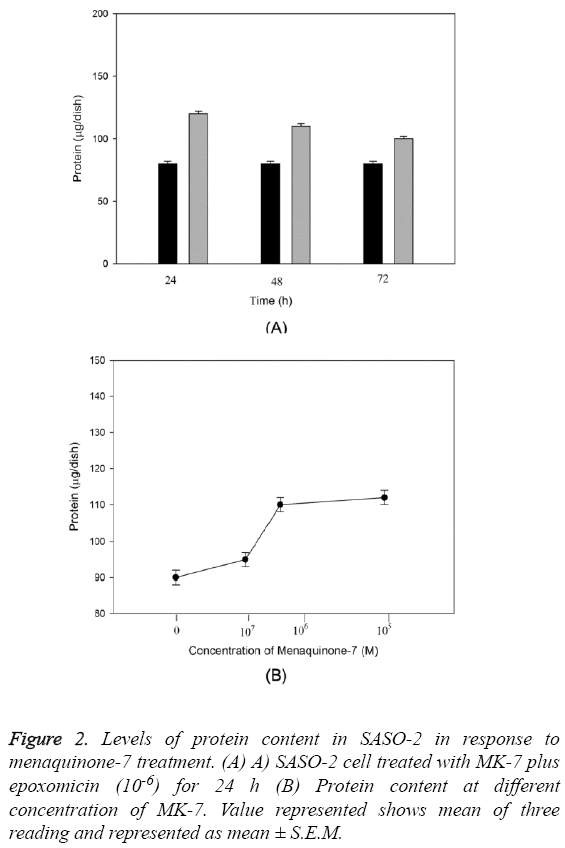
The anabolic effect of menaquinone-7 (MK-7) in osteoblastic SAOS-2 cells was examined. The effect of MK-7 on protein content in osteoblastic cells is shown in Figure 2. Cells were cultured for 24-72 h in a medium containing either epoxomicin or menaquinone-7 (10-6 M). The cells cultured with MK-7 for 24 or 48 h had higher protein content (Figure 2A). Such an increase was not seen in cells cultured for 72 h. When cells were cultured for 24 h in the presence of increasing concentrations of MK-7 (10-5 to 10-8) a significant increase in cellular protein content was seen with 10-6 and 10-5 M of MK-7 (P=0.0004) (Figure 2B).
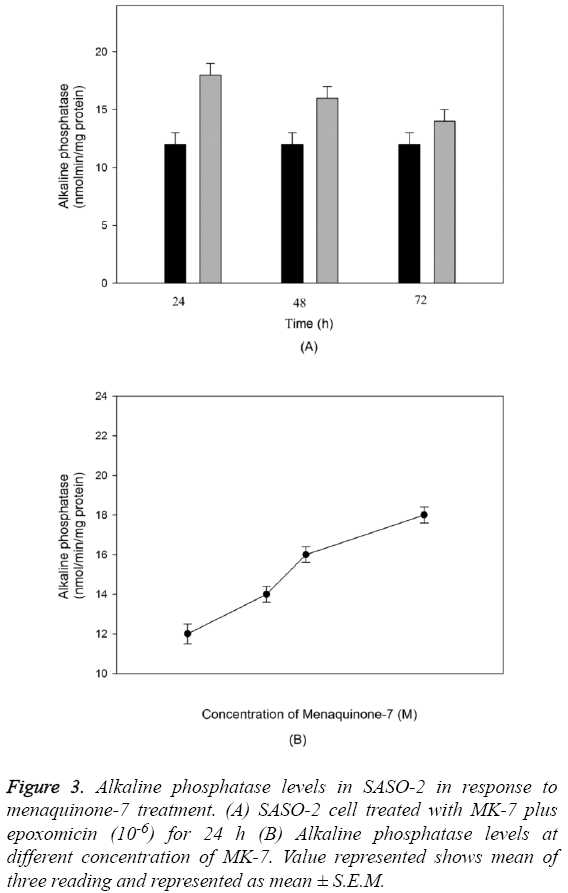
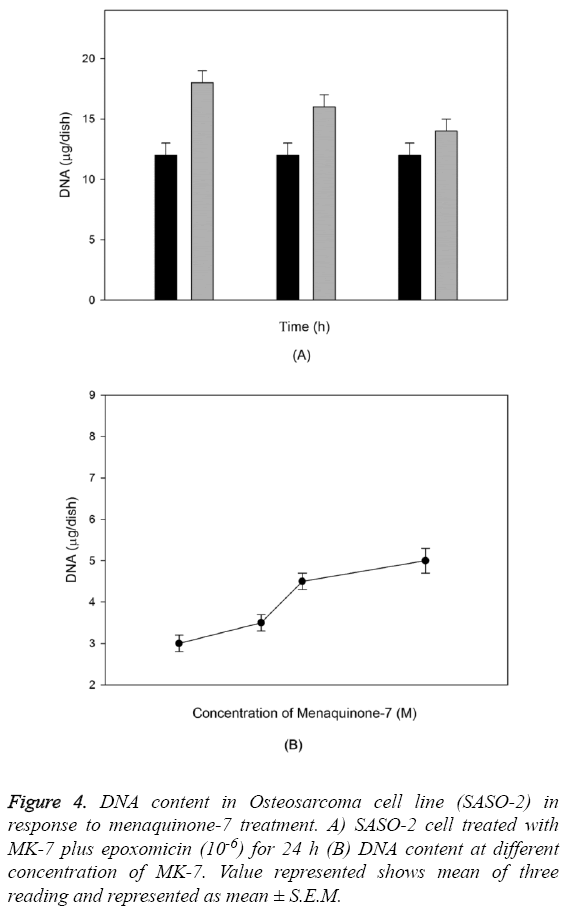
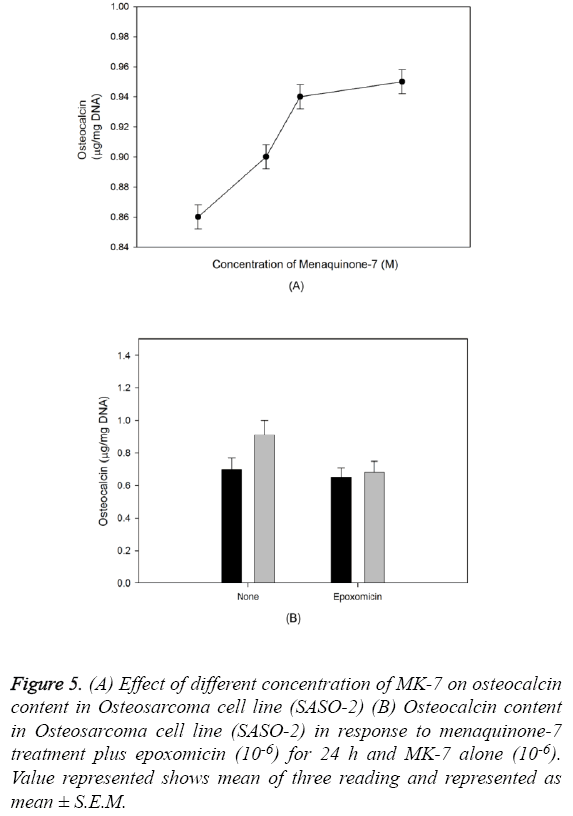
The outcome of MK-7 treatment on the levels of alkaline phosphatase in human osteoblast-like cells (SASO-2) is illustrated in Figure 3. There was an increase in the alkaline phosphatase levels due to MK-7 treatment [10-6] with increase in duration of treatment from 24 h to 72 h (P=0.0012). The increase in trend was also observed when cells were cultured at lower concentration. The effect of MK-7 on osteocalcin production in osteoblastic cells is shown in Figure 4. Cells were cultured for 24 h in a medium containing either vehicle or MK-7 (10-7-10-5 M). Osteocalcin content in culture medium was significantly increased in the presence of MK-7 (10-6 and 10-5 M) (Figures 4A and 4B). The effect of MK-7 (10-5 M) in increasing osteocalcin production in osteoblastic cells was completely prevented in the presence of epoxomicin (10-6 M) (Figures 5A and 5B). MK-7-treated SAOS-2 cells showed increased TRAP activity as compared with control cells. The number of TRAP-positive multinucleated cells was higher in the MK-7 treatment group than the control group (P=0.0007). TRAP-positive multinucleated cells were designated osteoclast-like cells (Figure 6).
Figure 3: Alkaline phosphatase levels in SASO-2 in response to menaquinone-7 treatment. (A) SASO-2 cell treated with MK-7 plus epoxomicin (10-6) for 24 h (B) Alkaline phosphatase levels at different concentration of MK-7. Value represented shows mean of three reading and represented as mean ± S.E.M.
Figure 5: (A) Effect of different concentration of MK-7 on osteocalcin content in Osteosarcoma cell line (SASO-2) (B) Osteocalcin content in Osteosarcoma cell line (SASO-2) in response to menaquinone-7 treatment plus epoxomicin (10-6) for 24 h and MK-7 alone (10-6). Value represented shows mean of three reading and represented as mean ± S.E.M.
Discussion
Hydroxyapatite is an important bone mineral and determines the strength of the bones. Osteoblasts are specialized mesenchymal stem cells that synthesize extremely dense, crosslinked collagen, as well as specialized proteins such as osteocalcin and osteopontin in small quantities, which compose the organic matrix of bone. Synthesis of osteocalcin is dependent on vitamin K and is involved in hydroxyapatite binding and bone formation. Hydroxyapatite has limited binding towards noncarboxylated osteocalcin [2,3], and therefore, vitamin K-dependent γ-carboxylation of osteocalcin is necessary. Vitamin K2 (menaquinone), a type of Vitamin K, has been shown to be important in bone metabolism. Menaquinone-4 (MK-4), a vitamin K2 with four isoprene units, has been found to be useful in the treatment of osteoporosis [6,8]. Natural MK-7, a vitamin K2 with seven isoprene units, is present in high quantities in fermented soybeans [10]. Similar to MK-4, MK-7 has been found to increase bone calcium content in vitro [9,10]. Supplementing rats with MK-7 has been shown to prevent ovariectomy-induced bone loss [11,12,14]. This suggests that MK-7 has preventive and therapeutic effects on osteoporosis. However, the cellular mechanism by which MK-7 affects bone metabolism in vivo is unclear and few studies have explored the mechanism. In our study, we showed that MK-7 can directly stimulate osteoblastic bone formation in vitro.
Our study showed that MK-7 had an anabolic effect on bone components and that it may be able to stimulate bone formation and calcification. It increased the DNA content, calcium content, and alkaline phosphatase activity in vitro in femoral-diaphyseal and -metaphyseal tissues of young rats. Additionally, epoxomicin, a protein synthesis inhibitor, completely inhibited the activity of MK-7, suggesting that the MK-7 was involved in the synthesis of new protein components. MK-7 significantly increased the production of osteocalcin in osteoblastic SAOS-2 cells in vitro, suggesting that MK-7 can directly stimulate the function of osteoblasts. The ability of MK-7 to increase DNA content in osteoblastic cells is shown by its ability to stimulate the proliferation of these cells. Additionally, MK-7 increased the activity of alkaline phosphatase, an osteoblast marker, suggesting that it may stimulate osteoblastic cell differentiation. These effects of MK-7 in osteoblastic cells were completely blocked by epoxomicin, which suggested that the activity of MK-7 was based on newly synthesized protein components. Moreover, epoxomicin also inhibited the MK-7-induced increase in osteocalcin production in osteoblastic cells.
Our study also suggested that MK-7 stimulates protein synthesis in osteoblastic cells. It may be possible that MK-7 acts on a common step of protein synthesis in translational process; however, the molecular mechanism of MK-7 is unclear and needs to be elucidated. Previous studies have reported that MK-7 increases the γ-carboxylation of osteocalcin in the serum of normal and ovariectomized rats [11]. Our study provides further evidence that MK-7 may stimulate bone mineralization by increasing γ-carboxylated osteocalcin in osteoblasts. The activities of MK-7 were blocked by a protein synthesis inhibitor, suggesting that MK-7 can stimulate bone mineralization by increasing protein synthesis in osteoblastic cells.
Conclusion
In conclusion, we demonstrated that MK-7 has an anabolic effect on bone components and can stimulate osteoblastic bone formation. It increased the activity of osteogenesis markers in the femoral-diaphyseal and -metaphyseal tissues of rats in vitro and directly stimulated biochemical functions in osteoblastic SAOS-2 cells in vitro, supporting the view that MK-7 can be used as a therapeutic agent for bone disease. Use of nonprimate model can be considered as a limitation of this study; however, a clinical study would be the best validation of the effectiveness of vitamin K in bone formation.
References
- Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A 1975; 72: 3925-3929.
- Price PA. Vitamin K-dependent formation of bone Gla protein (osteocalcin) and its function. Vitam Horm 1985; 42: 65-108.
- Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry 1982; 21: 2538-2547.
- Knapen MH, Hamulyak K, Vermeer C. The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion. Ann Intern Med 1989; 111: 1001-1005.
- Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J, Sambrook PN, Dodds RA, Bitensky L, Chayen J. Electrochemical detection of depressed circulating levels of vitamin K1 in osteoporosis. J Clin Endocrinol Metab 1985; 60: 1268-1269.
- Okano T. A New Horizon in Vitamin K Research. Yakugaku zasshi 2016; 136: 1141.
- Gundberg CM. Vitamin K and bone: past, present, and future. J Bone Miner Res. 2009; 24: 980-982.
- Akiyama Y, Hara K, Ohkawa I, Tajima T. Effects of menatetrenone on bone loss induced by ovariectomy in rats. Jpn J Pharmacol 1993; 62: 145-153.
- Ehara Y, Takahashi H, Hanahisa Y, Yamaguchi M. Effect of vitamin K2 (menaquinone-7) on bone metabolism in the femoral-metaphyseal tissues of normal and skeletal-unloaded rats: enhancement with zinc. Res Exp Med (Berl) 1996; 196: 171-178.
- Wu WJ, Kim MS, Ahn BY. The inhibitory effect of vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food Funct 2015; 6: 3351-3358.
- Yamaguchi M, Taguchi H, Gao YH, Igarashi A, Tsukamoto Y. Effect of vitamin K2 (menaquinone-7) in fermented soybean (natto) on bone loss in ovariectomized rats. J Bone Miner Metab 1999; 17: 23-29.
- Yamaguchi M, Kakuda H, Gao YH, Tsukamoto Y. Prolonged intake of fermented soybean (natto) diets containing vitamin K2 (menaquinone-7) prevents bone loss in ovariectomized rats. J Bone Miner Metab 2000; 18: 71-76.
- Tsukamoto Y. Studies on action of menaquinone-7 in regulation of bone metabolism and its preventive role of osteoporosis. Biofactors 2004; 22: 5-19.
- Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M. Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and gamma-carboxylated osteocalcin concentration in normal individuals. J Bone Miner Metab 2000; 18: 216-222.
- Seo H-J, Cho Y-E, Kim T, Shin H-I, Kwun I-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract 2010; 4: 356-361.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951 November; 193: 265-275.
- Ceriotti G. Determination of nucleic acids in animal tissues. J Biol Chem 1955 May; 214: 59-70.
- Sugimoto E, Yamaguchi M. Stimulatory effect of Daidzein in osteoblastic MC3T3-E1 cells. Biochem Pharmacol 2000; 59: 471-475.