ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 6
Vitrification and slow cryopreservation differentially affects oocyte survival, development and cytoskeleton status
1Center for Reproductive Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200135, PR China
2Shanghai Key laboratory for Assisted Reproduction and Reproductive Genetics, Shanghai 200135, PR China
3Animal husbandry and veterinary college, Shenyang agricultural university, Shenyang, Liaoning, PR China
- *Corresponding Author:
- Yan Hong
Center for Reproductive Medicine
Renji Hospital, School of Medicine
Shanghai Jiao Tong University, PR China
Accepted date: November 09, 2016
Backgroud: Oocyte cryopreservation is critical in assisted reproduction techniques. However, the effect of cryopreservation on the oocyte has not been widely explored yet. Currently a thorough understanding of oocyte cryobiology is urgently required due to its far lag behind the forces propelling the clinical application of oocyte cryopreservation.
Methods: In this study, we used two different ways including vitrification freezing and slow freezing to cryopreserve the mouse oocytes and elucidated how the cyro-procedures affected the survival, development potential and cytoskeleton of oocytes. The development of oocytes was evaluated on the formation of 2-cell and blastocyst. The cytoskeleton was determined by the immunostaining for the biomarker of Spindle and chromosome.
Results: The comparison between two different freezing methods indicated that the survival rate of oocyte at MII stage did not show significant difference between two methods, while the survival rate at GV stage was higher by vitrification than slow freezing. The development potential of oocytes cryopreserved at MII stage was different between two methods in the blastocyst ratio. Significant difference was found in ratio of mature oocyte, fertilized oocyte and blastocyst between two methods. Furthermore, the oocyte cytoskeleton status was better by vitrification freezing than slow freezing method. Besides, the comparison between the oocyte at two different stages only showed difference in mature oocyte ratio and cytoskeleton status by slow freezing method.
Conclusion: This study may explain why the oocyte cryopreservation success rate is as yet far from satisfactory and why cryopreserved oocytes should be treated differently from their fresh siblings. A fresh look at the characteristic features of oocytes after cryopreservation may work as a stimulus to further improvement of oocyte cryopreservation.
Keywords
Oocyte; Slow freezing; Vitrification freezing; Maturation; Fertilization; Cytoskeleton; Blastocyst.
Introduction
In the assisted reproductive technology (ART), oocyte cryopreservation has become a fertility preservation option for women [1]. Over the last decade, several methods for cryopreservation have been used in ART treatments. Two main methods are vitrification and slow freezing. The traditional option used for human ovarian cortex cryopreservation is slow freezing [2]. Slow freezing is an equilibration method exchanging the fluid between intracellular and extracellular spaces [3]. It results in freezing without degrading and deforming the cells had produced very little toxicity and osmotic damage. However, one of the obvious disadvantages of slow freezing is to cause the ice crystal formation [4]. Recently, vitrification freezing, an alternate cryopreservation technique, has gained more attention because it maintains good quality and functionality of ovarian tissues [5].
Vitrification is a non-equilibrium method of cryopreservation. It transforms cells into an amorphous glassy state so that it prevents the formation of ice crystals [6]. In contrast to the study on the cryopreservation of ovarian tissue and whole embryo, the research on the oocyte cryopreservation is still lagging behind [7]. This is probably due to the lower success rate in ART when using the frozen oocytes.
The cell survival after freezing depends on the multiple factors, including biochemical and physical cellular properties. Since the oocyte has the delicate meiotic spindle and the cytoplasm contains a high proportion of water, ice crystal formation lead to the lower survival rate after freezing and thawing [8]. Also, cryopreservation of mature oocytes may lead to hardening of the zona pellucida (ZP) and then impairing the fertilization [9]. Recently, using smaller amounts of vitrification solution have recently been reported [10,11]. Using this method, improved developmental rates of thawed bovine oocytes have been observed. In order to evaluate the effect of different freezing methods on cryopreserved ovarian tissue or oocytes, it was reported that vitrification of cleavage stage day 3 embryos induced higher live birth rates compared to the slow freezing [12]. However, systematic comparison elucidating the effect of cryopreservation on oocytes is still lacking.
In this study, we compared the effect of vitrification and slow freezing on the survival rate of oocytes cryopreserved at different stages. We also showed that different freezing methods also affected the maturation and development of oocytes. Furthermore, spindle and chromosome status in oocytes was differentially changed after cryopreservation. Collectively, this work provided a fresh look at the characteristic features of oocytes after cryopreservation, potentially working as a stimulus to further improvement of oocyte cryopreservation as well as the advancement of assisted reproductive technology.
Methods and Materials
Animals
All the animals used in this study were KunMing mice. The female mice were purchased from Shanghai SLAC Laboratory Animal Co., and the male mice were purchased from Wenzhou Medical School Animal Center.
GV oocyte collection
Oocytes were collected from adult (8-12 weeks of age) female mice. Superovulation was induced by 10 IU pregnant mare serum gonadotropin (PMSG). Germinal vesicle (GV) oocytes were obtained from ovaries and fallopian tubes. The cells were cultured in MEM medium overnight before cryopreservation.
In vitro maturation
GV oocytes were cultured in MEM medium for 24 hours. The drops were covered by mineral oil. In vitro maturated metaphase 2 (MII) oocytes were inseminated and the fertilized embryos assessed until the hatching blastocyst stage.
MII Oocyte collection
Superovulation was induced by 10 IU PMSG and 5 IU human chorionic gonadotropin (HCG).The oocytes were obtained from ovaries cultured in L-15 with 10% fetal bovine serum. Cumulus -oocyte complex (COC) were cultured in MEM medium. Cumulus cells were dispersed by 0.1 mg/ml hyaluronidase. Mature oocytes were cultured in MEM medium for 24 hours before freezing.
Vitrification freezing and thawing
Oocytes subjected to cryopreservation were sequentially washed in PBS with 20% FBS, SI with 10% EG and 10% DMSO, and St with 20% EO and 20% DMSO. Then, the cells were transferred to holding medium (TCM199 + 20% FBS), and subjected to sequential equilibration in vitrification solution 1 (VS1) and VS2. After equilibration, oocytes were loaded into SOPS freezing tubes and immediately immersed in liquid nitrogen. For the thawing, the cryopreserved oocytes were transferred to S1 with sucrose at room temperature for 3 mins. Then the oocytes were moved to S1 at room temperature for 3 min and gradually increased the temperature to 37°C. The thawed oocytes were cultured in MEM medium for 1-2 hours before the further experiment.
Slow freezing and thawing
Oocytes were slowly frozen in the cryoprotectant composed of MEM supplemented with 10% FBS, 0.1 mol/L sucrose and 20% DMSO. Oocytes were first equilibrated in the cryoprotectant solution for 30 min at 4°C with slow shaking. Then, 5-6 oocytes were transferred into a 1.8 mL cryovial containing 1 mL cryoprotectant solution. The vials were cooled in a programmable freezer. The oocytes were cooled from 4°C at −1°C/min to −9°C, and equilibrated for 6 min at −9°C. Then, the vials were cooled at −0.3°C/min to −40 °C. The vials were immersed into liquid nitrogen. For the thawing, the vials were put into 30°C water bath for 5 min. Then, the oocytes were sequentially washed in St with sucrose and PROH at room temperature for 5 min. The temperature was increased to 37°C slowed and stayed for 5 min. The oocytes were cultured in MEM medium for 1-2 hours before further experiments.
In vitro fertilization
The fertilization medium (KSOM with 4 mg/ml BSA) was preheated and equilibrated in incubator overnight. Adult (12-14 weeks of age) male mice were used for sperm collection. Spermatozoa suspension collected from epididymis was held for 30 min in fertilization medium. Then, the sperms were cultured in incubator for 30 min. Recovered oocytes were incubated with spermatozoa for 6 hours in fertilization medium. Zygotes were cultured in KSOM supplemented with 1 mg/ml BSA in a humidified atmosphere of 5% CO2 at 37 °C. Embryo development was observed 24 and 96 h after fertilization to calculate the percentage of fertilization and blastocyst formation, respectively.
Immnuostaining
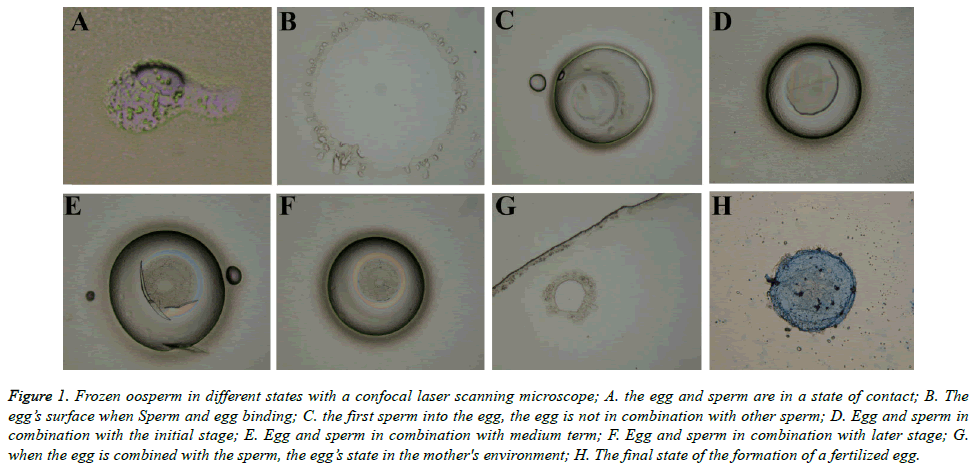
Cells were fixed with 4% paraformaldehyde for 40 mins and then permeabilized with 0.5% Triton X-100 for 15 minutes. Then, the cells were blocked in 1% BSA in PBS containing 0.1% Triton X-100 for 1 h. Then samples were incubated with mouse anti-TUBB antibody (Abcam) at 4°C overnight. FITCconjugated secondary antibody was used to visualize the positive signals. Chromosomes were stained with DAPI (Roche) for 10 min. After staining, samples were mounted on glass slides using vectashield mounting medium (Vector Labs) and examined with a confocal laser scanning microscope (Leica) (Figure 1).
Figure 1. Frozen oosperm in different states with a confocal laser scanning microscope; A. the egg and sperm are in a state of contact; B. The egg’s surface when Sperm and egg binding; C. the first sperm into the egg, the egg is not in combination with other sperm; D. Egg and sperm in combination with the initial stage; E. Egg and sperm in combination with medium term; F. Egg and sperm in combination with later stage; G. when the egg is combined with the sperm, the egg’s state in the mother's environment; H. The final state of the formation of a fertilized egg.
Statistical analysis
Data are presented as mean ± SEM. Survival rate was calculated according to the following formula: survival rate=number of surviving oocytes/number of recovered oocytes × 100%. The maturation rate was calculated as number of oocytes with first polar bodies/number of GV oocytes × 100%. The fertilization rate was calculated as number of twocell zygotes/number of surviving oocytes × 100%. The blastocyst formation was calculated as number of blastocysts/ number of surviving oocytes × 100%. The significant difference was evaluated using unpaired student’s t tests. A pvalue <0.05 was considered statistically significant.
Results
Comparison of oocyte survival rate between two different freezing methods
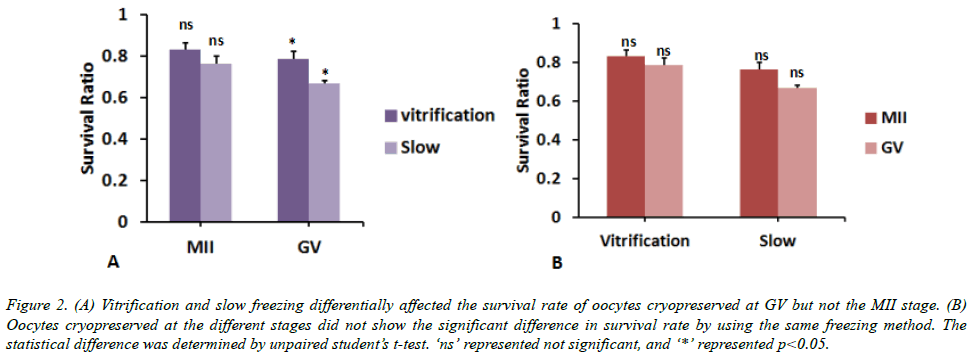
Firstly, we compared the survival rate between vitrification and slow oocyte freezing. We found that the survival rate of oocyte at MII stage did not show significant difference between two methods. The oocyte survival rate by vitrification freezing is 108 ± 2% of that by slow freezing (p>0.05, Figure 2A). By contrast, the survival rate at GV stage was higher by 17 ± 3% when using vitrification freezing than slow freezing (p<0.05, Figure 1A). Besides, there was no significant difference in the survival rate of oocytes cryopreserved at different stages when using the same freezing method (p>0.05, Figure 2B). These data indicated that vitrification freezing is better than slow freezing in GV stage oocyte survival rate.
Figure 2. (A) Vitrification and slow freezing differentially affected the survival rate of oocytes cryopreserved at GV but not the MII stage. (B) Oocytes cryopreserved at the different stages did not show the significant difference in survival rate by using the same freezing method. The statistical difference was determined by unpaired student’s t-test. ‘ns’ represented not significant, and ‘*’ represented p<0.05.
Comparison of oocyte developmental potential between two different freezing methods
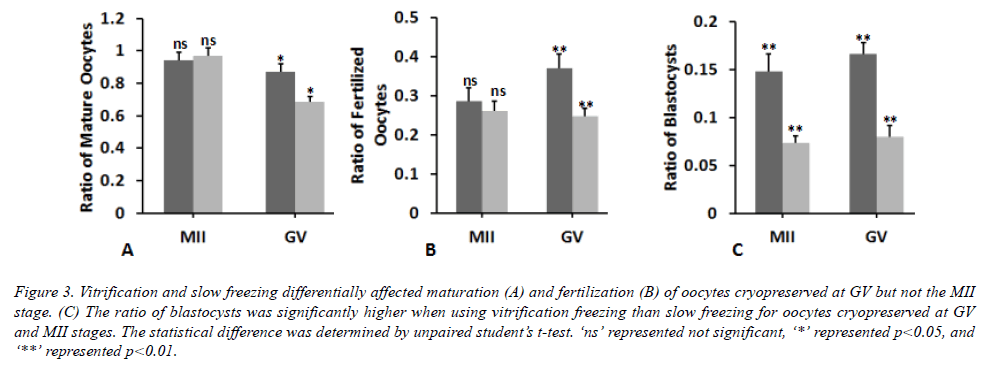
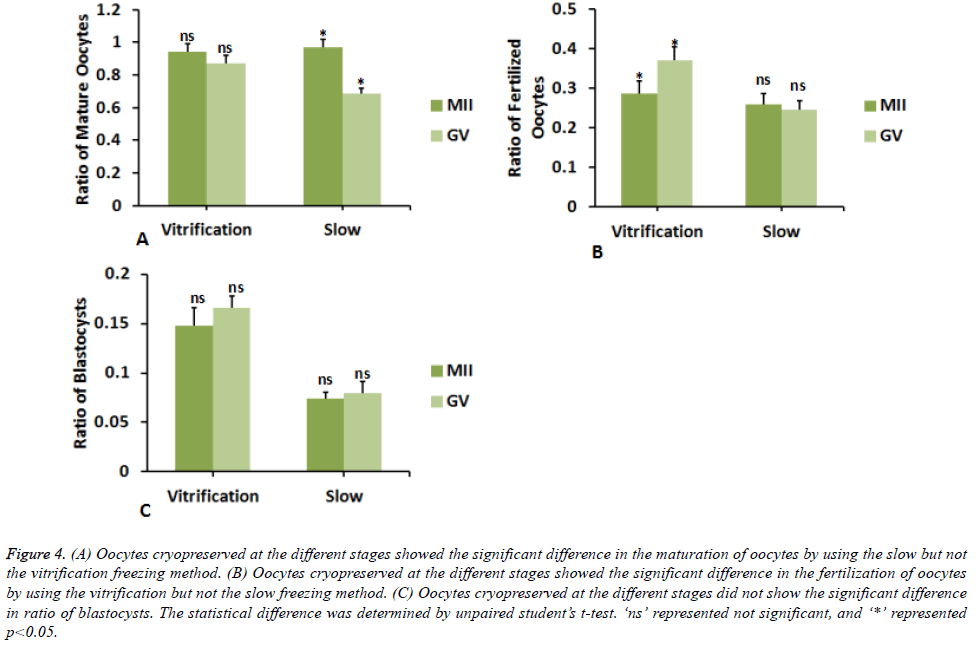
Then, we determined whether the two different freezing methods differentially affected the developmental potential of oocytes cryopreserved at different stages. We found that different freezing method did not lead to significant difference in the maturation and fertilization of oocytes cryopreserved at MII stage (Figures 3A and 3B). By contrast, the blastocyst ratio was significantly decreased by 49 ± 8% in slow freezing compared to the vitrification freezing for oocytes at MII stage (Figure 3C). Cryopreservation of oocyes at GV stage caused significant difference between slow and vitrification freezing vitrification freezing. The ratios of mature oocytes, fertilized oocytes and blastocysts were significantly lowered by 21 ± 2%, 32 ± 7%, and 52 ± 8% respectively when using slow freezing compared to the vitrification freezing. On the other way, we observed the apparent difference in the fertilization (Figure 4B) but not the maturation and development of oocytes cryopreserved at MII and GV stages (Figures 3A and 4C) by vitrification freezing. As to the slow freezing, significant difference was only found in the ratio of mature oocytes cryopreserved at MII and GV stages (Figure 3A), but not in the ratio of fertilized oocytes or blastocysts (Figures 4B and 4C). Collectively, these data showed that different freezing methods could affect the developmental potential of cyropreserved oocytes, while cyropreserving oocytes at different stages by the same method did not lead to apparent difference in the developmental potential.
Figure 3. Vitrification and slow freezing differentially affected maturation (A) and fertilization (B) of oocytes cryopreserved at GV but not the MII stage. (C) The ratio of blastocysts was significantly higher when using vitrification freezing than slow freezing for oocytes cryopreserved at GV and MII stages. The statistical difference was determined by unpaired student’s t-test. ‘ns’ represented not significant, ‘*’ represented p<0.05, and ‘**’ represented p<0.01.
Figure 4. (A) Oocytes cryopreserved at the different stages showed the significant difference in the maturation of oocytes by using the slow but not the vitrification freezing method. (B) Oocytes cryopreserved at the different stages showed the significant difference in the fertilization of oocytes by using the vitrification but not the slow freezing method. (C) Oocytes cryopreserved at the different stages did not show the significant difference in ratio of blastocysts. The statistical difference was determined by unpaired student’s t-test. ‘ns’ represented not significant, and ‘*’ represented p<0.05.
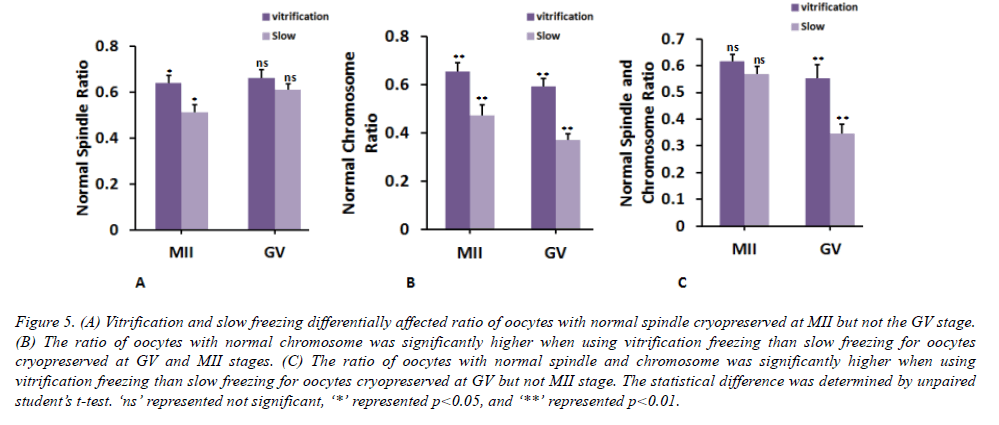
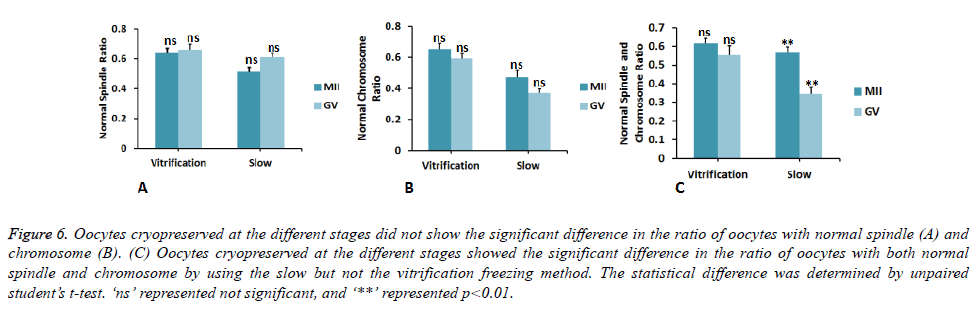
Comparison of oocyte cytoskeleton between two different freezing methods
Next, we compared the cytoskeleton in oocytes cryopreserved by different methods. At MII stage, ratio of oocytes having normal spindle and chromosome was significantly decreased by 26 ± 8% and 39 ± 5% using slow freezing compared to those by vitrification freezing (Figures 5A and 5B). Similarly, at GV stage, cytoskeleton of oocyte was also significantly affected. The ratio of oocytes having normal chromosome and spindle & chromosome was significantly decreased by 59 ± 7% and 60 ± 11% using slow freezing compared to those by vitrification freezing (Figures 4B and 4C). By using the vitrification freezing, there was no obvious difference in the ratio of oocytes with normal cytoskeleton between MII and GV stages (Figure 6). By contrast, the ratio of oocytes with normal cytoskeleton was lower by 39 ± 6% when these oocytes were cryopreserved at GV stage, compared to oocytes cryopreserved at the MII stage (Figure 6C). This demonstrated that different freezing methods will preferentially affect the cytoskeleton of oocyte cryopreserved at GV stage than in MII stage.
Figure 5. (A) Vitrification and slow freezing differentially affected ratio of oocytes with normal spindle cryopreserved at MII but not the GV stage. (B) The ratio of oocytes with normal chromosome was significantly higher when using vitrification freezing than slow freezing for oocytes cryopreserved at GV and MII stages. (C) The ratio of oocytes with normal spindle and chromosome was significantly higher when using vitrification freezing than slow freezing for oocytes cryopreserved at GV but not MII stage. The statistical difference was determined by unpaired student’s t-test. ‘ns’ represented not significant, ‘*’ represented p<0.05, and ‘**’ represented p<0.01.
Figure 6. Oocytes cryopreserved at the different stages did not show the significant difference in the ratio of oocytes with normal spindle (A) and chromosome (B). (C) Oocytes cryopreserved at the different stages showed the significant difference in the ratio of oocytes with both normal spindle and chromosome by using the slow but not the vitrification freezing method. The statistical difference was determined by unpaired student’s t-test. ‘ns’ represented not significant, and ‘**’ represented p<0.01.
Discussion
Over the last decade, several methods for cryopreservation have been used in ART treatments. The traditional slow freezing, which results in freezing without degrading and deforming the cells with little toxicity and osmotic damage, suffered from the ice crystal formation [3]. While a new vitrification freezing method is considered as a method which can maintains good quality and functionality of ovarian tissues [5]. Despite the numerous progresses made in the cryopreservation techniques in ART, the freezing methods applied in the oocyte cryopreservation and their effect on the cellular property of oocytes is still elusive to us. Therefore, this study provided a clear elucidation that different freezing methods could differentially affect the survival, maturation, and development of oocytes. Our results showed that the survival rate of GV oocytes was higher in vitrification than the slow freezing (Figure 2A). This is probably due to the apoptotic alteration in the oocytes cryopreserved at different stages. Previous study reported that slow freezing could initiate the lower expression level of anti-apoptotic genes and higher expression level of pro-apoptotic genes, compared to the vitrification [13]. This conclusion may explain when the oocyte survival rate was differential between slow and vitrification freezing in our study. We also found that the developmental potential was affected by different freezing mathods. The blastocyst ratio in the vitrification group was much higher than the slow freezing group (Figure 3C). Formation of blastocyst is a critical step in the pregnancy and afterwards the embryo will undergo implantation, establishing the connection between the mother and embryo [14]. Therefore, studying the effect on cryopreservation on the blastocyst formation is of great importance to increase the success rate of ART application. In the future work, the molecular mechanism involved in the differential ratio of blastocyst formation will be emphasized. Previous reports indicated that OCT4 promoter methylation profile [15], IGF2 and Glut3 [16] exerted effect on the blastocyst formation in vitrification freezing. These are important factors to be studied in the future work regarding the mechanism of alternation in blastocyst formation after oocyte cryopreservation.
Acknowledgment
The study is funded by Shanghai Key laboratory for Assisted Reproduction and Reproductive Genetics (12DZ2260600).
References
- Tucker MJ, Morton PC, Wright G, Sweitzer CL, Massey JB. Clinical application of human egg cryopreservation. Hum Reprod 1998; 13: 3156-3159.
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation, Fertil Steril 2013; 99: 1503-1513.
- Mazur P. Equilibrium, Quasi-Equilibrium, and Nonequilibrium Freezing of Mammalian Embryos, Cell Biophy 1990; 17: 53-92.
- Gautam SK, Verma V, Singh B, Palta P, Singla SK, Chauhan MS, Manik RS. Effect of slow freezing on morphology and developmental competence of buffalo (Bubalus bubalis) immature oocytes, Ani Reprod Sci 2008; 105: 311-318.
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue, Reprod Biomed Onl 2009; 18: 568-577.
- Wowk B, Leitl E, Rasch CM, Mesbah-Karimi N, Harris SB. Vitrification enhancement by synthetic ice blocking agents. Cryobiol 2000; 40: 228-236.
- Practice Committees of American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013; 99: 37-43.
- Coticchio G, Bonu MA, Sciajno R, Sereni E, Bianchi V. Truths and myths of oocyte sensitivity to controlled rate freezing. Reprod Biomed Online 2007; 15: 24-30.
- Matson PL, Graefling J, Junk SM, Yovich JL, Edirisinghe WR. Cryopreservation of oocytes and embryos: use of a mouse model to investigate effects upon zona hardness and formulate treatment strategies in an in-vitro fertilization programme. Hum Reprod 1997; 12: 1550-1553.
- Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, Zhou P. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril 2009; 92: 1306-1311.
- Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenol 2007; 67: 73-80.
- Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D'Hooghe TM, Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT, Human Reprod 2015; 30: 1820-1830.
- Taghavi SA, Valojerdi MR, Moghadam MF, Ebrahimi B. Vitrification of mouse preantral follicles versus slow freezing: Morphological and apoptosis evaluation, Ani Sci J 2015; 86: 37-44.
- Zhang S, Lin H, Kong S, Wang S, Wang H. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013; 34: 939-980.
- Saenz-De-Juano MD, Penaranda DS, Marco-Jimenez F, Vicente JS. Does vitrification alter the methylation pattern of OCT4 promoter in rabbit late blastocyst. Cryobiol 2014; 69: 178-180.
- Zander-Fox D, Cashman KS, Lane M. The presence of 1 mM glycine in vitrification solutions protects oocyte mitochondrial homeostasis and improves blastocyst development. J Assist Reprod Gen 2013; 30: 107-116.